Chapter First Online: 02 April 2021,Progress in the Chemistry of Organic Natural Products 114 pp 313-337
Natural products are a class of chemical compounds that are biosynthesized by living organisms, including humans. Endogenous natural products are produced by human cells as well as by the human microbiome. This contribution describes the current understanding and recent progress made on endogenous natural products that are produced by human cells, including amines, steroids, and fatty acid-derived natural products. The co-metabolism and natural product produced by the human microbiome will also be described, including the involvement of tryptophan, bile acids, choline, and cysteine. New strategies and technologies have been introduced that can be applied to identify and characterize those natural products produced by the human microbiome in terms of their composition and physiological function.
Plant metabolites play important roles in both plant physiology and drug discovery. Taking advantage of new emerging technologies such as next generation sequencing (NGS), whole genome assembly, bioinformatics, omics-based strategies have been demonstrated as popular and powerful ways to elucidate complex metabolic pathways in plants. In this viewpoint, biosynthetic intermediates probes have been proposed as the potentinal tools to study the plant natural product biosynthesis via chemical proteomics appoaches or transcriptome analysis.
Jinyi Bai , Fusheng Guo , Mengyao Li , Yulong Li * and Xiaoguang Lei* Received 6th January 2021 , Accepted 18th March 2021 First published on 20th March 2021
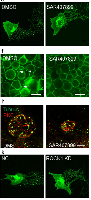
We here describe a fluorescent signal amplification method termed “Click-based amplification” that can be well integrated with various click-labelling modes, including chemical labelling, genetic incorporation and covalent inhibitor probe mediated target labelling. Picolyl azide (pAz) was used as a functional group of a streptavidin-based amplifier to enhance the efficiency of click chemistry. Click-based amplification provided 3.0–12.7 fold amplification on fixed HeLa cells with different click-labelling modes. Click-based amplification has proven to be superior to tyramide signal amplification (TSA) in view of its low nonspecific amplification and high signal-to-noise ratio. Moreover, in terms of the challenging signal amplification of tissue specimens, Click-based amplification successfully achieved remarkable fluorescence enhancement on intestinal tissue slices of afatinib-N3 treated mice, which provided direct evidence of the presence of afatinib-N3 in the intestinal tissues and helped in revealing the off-target toxicity of afatinib. Collectively, these results illustrate that Click-based amplification could serve as a promising method for bioimaging studies
Polycyclic natural products are an inexhaustible source of medicinal agents, and their complex molecular architecture renders challenging synthetic targets where innovative and effective approaches for their rapid construction are urgently required. The total synthesis of polycyclic natural products has witnessed exponential progression along with the emergence of new synthetic strategies and concepts, such as sequential C−H functionalizations, radical-based transformations, and functional group pairing strategies. Our group exerts continued interest in the construction of bioactive and structurally complex natural products as well as evaluation of the mode of action of these molecules. In this Account, we will showcase how these new synthetic strategies are employed and guide our total synthesis endeavors. During the last two decades, a series of remarkable advances in C−H functionalization have led to the emergence of many new approaches to directly functionalize C−H bonds into useful functional groups. These selective transformations have provided a great opportunity for the step- and atom-economical construction of key fragments in complex molecule synthesis. We recently furnished the total syntheses for polycyclic natural products: incarviatone A, chrysomycin A, polycarcin V, and gilvocarcin V by employing a multiple C−H bond functionalization strategy. The polysubstituted benzene or naphthalene skeleton was constructed through sequential and site-selective C−H functionalizations from readily available simple starting materials, which reduced the number of steps and streamlined synthesis.
The rise of resistance against all known antibiotics is a global crisis. There is an urgent need to develop rapid and sensitive diagnostic methods to detect pathogenic bacteria in clinical samples. Pseudopaline, a metallophore produced by the human pathogen Pseudomonas aeruginosa, transports divalent metal ions via a dedicated active transport system, making it an ideal carrier for a second functional moiety. In this work, we have developed a fluorescein-labeled pseudopaline probe (P-FL), which could specifically detect P. aeruginosa in samples (in vitro) with many other bacterial species, mammalian cells, or mouse stomach tissue sections. By replacing the fluorescein with the near-infrared fluorophore Cy-7 (P-Cy7), we showed that P. aeruginosa infections could also be specifically detected in a mouse model (in vivo). The remarkable selectivity of these pseudopaline fluorescent probes is because the pseudopaline-mediated metal transport system is specific to P. aeruginosa only. Therefore, our results show that pseudopaline based probes may provide a new way to develop fast and effective diagnostics of P. aeruginosa infections.
Xiaojing Liu, Jun Yang, Lei Gao, Liyun Zhang, and Xiaoguang Lei
Diels–Alder reaction is one of the most important transformations used in organic synthesis, with the ability to construct two new C–C bonds and up to four chiral centers simultaneously. However, the biggest synthetic challenge in Diels–Alder reaction lies in controlling its regio-, diastereo-, and enantioselectivity.Using Stille cross-coupling and enzymatic Diels–Alder reaction as the key steps, the first chemoenzymatic total synthesis of artonin I is achieved in 30% overall yield over only seven steps. This enzymatic Diels–Alder reaction catalyzed by MaDA is featured with excellent endo- and enantioselectivity and high catalytic efficiency (kcat/KM = 362 ± 54 mm−1 s−1). These successful chemoenzymatic total syntheses of artonin I and dideoxyartonin I demonstrated the remarkable potential of the intermolecular Diels–Alderase MaDA in biocatalysis.
Weilong Liu, Zongwei Yue, Zhen Wang, Houhua Li, and Xiaoguang Lei
We report herein the diversity-oriented synthesis of various tetracyclic Isodon diterpenoid scaffolds guided by radical cyclization logic. Our substrate-based dienyne radical cyclization approach is distinctive from reagent-based rearrangement approaches that are generally applied in biosynthesis or previous synthetic studies. An unprecedented cyclization at C14 via 1,5- radical translocation/5-exo-trig cyclization is observed, which enriches our radical cyclization pattern. Furthermore, biological evaluations revealed that several new natural product-like compounds showed promising anticancer activities against various cancer cell lines.
Lei Gao, Cong Su, Xiaoxia Du, Ruishan Wang, Shuming Chen, Yu Zhou, Chengwei Liu, Xiaojing Liu, Runze Tian, Liyun Zhang, Kebo Xie, She Chen, Qianqian Guo, Lanping Guo, Yoshio Hano, Manabu Shimazaki , Atsushi Minami, Hideaki Oikawa, Niu Huang, K. N. Houk, Luqi Huang*, Jungui Dai* and Xiaoguang Lei*
Nature Chem. 2020, https://doi.org/10.1038/s41557-020-0467-7
The Diels–Alder reaction is one of the most powerful and widely used methods in synthetic chemistry for the stereospecific construction of carbon–carbon bonds. Despite the importance of Diels–Alder reactions in the biosynthesis of numerous secondary metabolites, no naturally occurring stand-alone Diels–Alderase has been demonstrated to catalyse intermolecular Diels– Alder transformations. Here we report a flavin adenine dinucleotide-dependent enzyme, Morus alba Diels–Alderase (MaDA), from Morus cell cultures, that catalyses an intermolecular [4+2] cycloaddition to produce the natural isoprenylated flavonoid chalcomoracin with a high efficiency and enantioselectivity. Density functional theory calculations and preliminary measurements of the kinetic isotope effects establish a concerted but asynchronous pericyclic pathway. Structure-guided mutagenesis and docking studies demonstrate the interactions of MaDA with the diene and dienophile to catalyse the [4+2] cycloaddition. MaDA exhibits a substrate promiscuity towards both dienes and dienophiles, which enables the expedient syntheses of structurally diverse natural products. We also report a biosynthetic intermediate probe (BIP)-based target identification strategy used to discover MaDA.
The β-lactam antibiotic resistance has become a critical global health threat. One of the major reasons for drug resistance is the expression of β-lactamases especially metallo-β-lactamases such as New Delhi metallo-β-lactamase (NDM-1) by Gram-negative bacteria. The fungal natural product aspergillomarasmine A (AMA) was found to be a promising inhibitor of NDM-1 to potentiate currently used β-lactam antibiotics to overcome drug resistance both in vitro and in vivo. Although several chemical synthesis and chemoenzymatic synthesis approaches to access AMA have been reported, the biosynthesis of AMA was still elusive. Herein, we identified the key enzyme responsible for the biosynthesis of AMA in Aspergillus oryzae. AMA synthase is a PLP-dependent cysteine synthase homologous protein which utilizes O-acetyl-Lserine/O-phospho-L-serine and L-aspartic acid as its substrates. Remarkably, this enzyme catalyzes two consecutive C-N bond formations to produce AMA efficiently which may be attributed to the spacious substrate-binding pocket. PLP is covalently bound to Lys61 by an internal aldimine from the PLP re face, and the si face of PLP pyridine ring is accessible to the substrates to promote the nucleophilic addition of amino acids to the double bond of the external adiminine and ultimately to generate chiral Cα with S configuration. The catalytic mechanism was proposed based on molecular docking and biochemical experiments. In addition, we have further investigated the substrate scope of AMA synthase and identified a variant enzyme which shows promising potential in producing structurally diverse molecules containing C-N bond.
-
-
-

-

-
-

-
-
-

-

Human Endogenous Natural Products
Yingjie BaiLiyun ZhangXiaoguang Lei*
Chapter
First Online: 02 April 2021,Progress in the Chemistry of Organic Natural Products 114 pp 313-337
Natural products are a class of chemical compounds that are biosynthesized by living organisms, including humans. Endogenous natural products are produced by human cells as well as by the human microbiome. This contribution describes the current understanding and recent progress made on endogenous natural products that are produced by human cells, including amines, steroids, and fatty acid-derived natural products. The co-metabolism and natural product produced by the human microbiome will also be described, including the involvement of tryptophan, bile acids, choline, and cysteine. New strategies and technologies have been introduced that can be applied to identify and characterize those natural products produced by the human microbiome in terms of their composition and physiological function.
Biosynthetic Intermediate Probes for Visualizing and Identifying the Biosynthetic Enzymes of Plant Metabolites
Lei Gao*and Xiaoguang Lei*
ChemBioChem, 2021, 22(6), pp. 982–984
Plant metabolites play important roles in both plant physiology and drug discovery. Taking advantage of new emerging technologies such as next generation sequencing (NGS), whole genome assembly, bioinformatics, omics-based strategies have been demonstrated as popular and powerful ways to elucidate complex metabolic pathways in plants. In this viewpoint, biosynthetic intermediates probes have been proposed as the potentinal tools to study the plant natural product biosynthesis via chemical proteomics appoaches or transcriptome analysis.
Click-based amplification: designed to facilitate various target labelling with ultralow background
Jinyi Bai , Fusheng Guo , Mengyao Li , Yulong Li * and Xiaoguang Lei*
Received 6th January 2021 , Accepted 18th March 2021
First published on 20th March 2021
We here describe a fluorescent signal amplification method termed “Click-based amplification” that can be well integrated with various click-labelling modes, including chemical labelling, genetic incorporation and covalent inhibitor probe mediated target labelling. Picolyl azide (pAz) was used as a functional group of a streptavidin-based amplifier to enhance the efficiency of click chemistry. Click-based amplification provided 3.0–12.7 fold amplification on fixed HeLa cells with different click-labelling modes. Click-based amplification has proven to be superior to tyramide signal amplification (TSA) in view of its low nonspecific amplification and high signal-to-noise ratio. Moreover, in terms of the challenging signal amplification of tissue specimens, Click-based amplification successfully achieved remarkable fluorescence enhancement on intestinal tissue slices of afatinib-N3 treated mice, which provided direct evidence of the presence of afatinib-N3 in the intestinal tissues and helped in revealing the off-target toxicity of afatinib. Collectively, these results illustrate that Click-based amplification could serve as a promising method for bioimaging studies
New Strategies in the Efficient Total Syntheses of Polycyclic Natural Products
Polycyclic natural products are an inexhaustible source of medicinal agents, and their complex molecular architecture renders challenging synthetic targets where innovative and effective approaches for their rapid construction are urgently required. The total synthesis of polycyclic natural products has witnessed exponential progression along with the emergence of new synthetic strategies and concepts, such as sequential C−H functionalizations, radical-based transformations, and functional group pairing strategies. Our group exerts continued interest in the construction of bioactive and structurally complex natural products as well as evaluation of the mode of action of these molecules. In this Account, we will showcase how these new synthetic strategies are employed and guide our total synthesis endeavors. During the last two decades, a series of remarkable advances in C−H functionalization have led to the emergence of many new approaches to directly functionalize C−H bonds into useful functional groups. These selective transformations have provided a great opportunity for the step- and atom-economical construction of key fragments in complex molecule synthesis. We recently furnished the total syntheses for polycyclic natural products: incarviatone A, chrysomycin A, polycarcin V, and gilvocarcin V by employing a multiple C−H bond functionalization strategy. The polysubstituted benzene or naphthalene skeleton was constructed through sequential and site-selective C−H functionalizations from readily available simple starting materials, which reduced the number of steps and streamlined synthesis.
A pseudopaline fluorescent probe for the selective detection of Pseudomonas aeruginosa
The rise of resistance against all known antibiotics is a global crisis. There is an urgent need to develop rapid and sensitive diagnostic methods to detect pathogenic bacteria in clinical samples. Pseudopaline, a metallophore produced by the human pathogen Pseudomonas aeruginosa, transports divalent metal ions via a dedicated active transport system, making it an ideal carrier for a second functional moiety. In this work, we have developed a fluorescein-labeled pseudopaline probe (P-FL), which could specifically detect P. aeruginosa in samples (in vitro) with many other bacterial species, mammalian cells, or mouse stomach tissue sections. By replacing the fluorescein with the near-infrared fluorophore Cy-7 (P-Cy7), we showed that P. aeruginosa infections could also be specifically detected in a mouse model (in vivo). The remarkable selectivity of these pseudopaline fluorescent probes is because the pseudopaline-mediated metal transport system is specific to P. aeruginosa only. Therefore, our results show that pseudopaline based probes may provide a new way to develop fast and effective diagnostics of P. aeruginosa infections.
Chemoenzymatic Total Syntheses of Artonin I with an Intermolecular Diels–Alderase
Xiaojing Liu, Jun Yang, Lei Gao, Liyun Zhang, and Xiaoguang Lei
Diels–Alder reaction is one of the most important transformations used in organic synthesis, with the ability to construct two new C–C bonds and up to four chiral centers simultaneously. However, the biggest synthetic challenge in Diels–Alder reaction lies in controlling its regio-, diastereo-, and enantioselectivity.Using Stille cross-coupling and enzymatic Diels–Alder reaction as the key steps, the first chemoenzymatic total synthesis of artonin I is achieved in 30% overall yield over only seven steps. This enzymatic Diels–Alder reaction catalyzed by MaDA is featured with excellent endo- and enantioselectivity and high catalytic efficiency (kcat/KM = 362 ± 54 mm−1 s−1). These successful chemoenzymatic total syntheses of artonin I and dideoxyartonin I demonstrated the remarkable potential of the intermolecular Diels–Alderase MaDA in biocatalysis.
Syntheses of Skeletally Diverse Tetracyclic Isodon Diterpenoid Scaffolds Guided by Dienyne Radical Cyclization Logic
Weilong Liu, Zongwei Yue, Zhen Wang, Houhua Li, and Xiaoguang Lei
We report herein the diversity-oriented synthesis of various tetracyclic Isodon diterpenoid scaffolds guided by radical cyclization logic. Our substrate-based dienyne radical cyclization approach is distinctive from reagent-based rearrangement approaches that are generally applied in biosynthesis or previous synthetic studies. An unprecedented cyclization at C14 via 1,5- radical translocation/5-exo-trig cyclization is observed, which enriches our radical cyclization pattern. Furthermore, biological evaluations revealed that several new natural product-like compounds showed promising anticancer activities against various cancer cell lines.
Chemical screening identifies ROCK1 as a regulator of migrasome formation
Puzhong Lu, Rui Liu, Di Lu, Yue Xu, Xueyi Yang, Zheng Jiang, Chun Yang, Li Yu*, Xiaoguang Lei* & Yang Chen*
Cell Discovery 2020, 6, 51
FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis
Lei Gao, Cong Su, Xiaoxia Du, Ruishan Wang, Shuming Chen, Yu Zhou, Chengwei Liu, Xiaojing Liu, Runze Tian, Liyun Zhang, Kebo Xie, She Chen, Qianqian Guo, Lanping Guo, Yoshio Hano, Manabu Shimazaki , Atsushi Minami, Hideaki Oikawa, Niu Huang, K. N. Houk, Luqi Huang*, Jungui Dai* and Xiaoguang Lei*
Nature Chem. 2020, https://doi.org/10.1038/s41557-020-0467-7
The Diels–Alder reaction is one of the most powerful and widely used methods in synthetic chemistry for the stereospecific construction of carbon–carbon bonds. Despite the importance of Diels–Alder reactions in the biosynthesis of numerous secondary metabolites, no naturally occurring stand-alone Diels–Alderase has been demonstrated to catalyse intermolecular Diels– Alder transformations. Here we report a flavin adenine dinucleotide-dependent enzyme, Morus alba Diels–Alderase (MaDA), from Morus cell cultures, that catalyses an intermolecular [4+2] cycloaddition to produce the natural isoprenylated flavonoid chalcomoracin with a high efficiency and enantioselectivity. Density functional theory calculations and preliminary measurements of the kinetic isotope effects establish a concerted but asynchronous pericyclic pathway. Structure-guided mutagenesis and docking studies demonstrate the interactions of MaDA with the diene and dienophile to catalyse the [4+2] cycloaddition. MaDA exhibits a substrate promiscuity towards both dienes and dienophiles, which enables the expedient syntheses of structurally diverse natural products. We also report a biosynthetic intermediate probe (BIP)-based target identification strategy used to discover MaDA.
Identification of the AMA Synthase from the Aspergillomarasmine A Biosynthesis and Evaluation of Its Biocatalytic Potential
Qianqian Guo, Dongshan Wu, Lei Gao, Yingjie Bai, Yuan Liu, Nianxin Guo, Xiaoxia Du, Jun Yang, Xiaoming Wang, and Xiaoguang Lei
ACS Catal. 2020, https://doi/10.1021/acscatal.0c01187
The β-lactam antibiotic resistance has become a critical global health threat. One of the major reasons for drug resistance is the expression of β-lactamases especially metallo-β-lactamases such as New Delhi metallo-β-lactamase (NDM-1) by Gram-negative bacteria. The fungal natural product aspergillomarasmine A (AMA) was found to be a promising inhibitor of NDM-1 to potentiate currently used β-lactam antibiotics to overcome drug resistance both in vitro and in vivo. Although several chemical synthesis and chemoenzymatic synthesis approaches to access AMA have been reported, the biosynthesis of AMA was still elusive. Herein, we identified the key enzyme responsible for the biosynthesis of AMA in Aspergillus oryzae. AMA synthase is a PLP-dependent cysteine synthase homologous protein which utilizes O-acetyl-Lserine/O-phospho-L-serine and L-aspartic acid as its substrates. Remarkably, this enzyme catalyzes two consecutive C-N bond formations to produce AMA efficiently which may be attributed to the spacious substrate-binding pocket. PLP is covalently bound to Lys61 by an internal aldimine from the PLP re face, and the si face of PLP pyridine ring is accessible to the substrates to promote the nucleophilic addition of amino acids to the double bond of the external adiminine and ultimately to generate chiral Cα with S configuration. The catalytic mechanism was proposed based on molecular docking and biochemical experiments. In addition, we have further investigated the substrate scope of AMA synthase and identified a variant enzyme which shows promising potential in producing structurally diverse molecules containing C-N bond.