Aspartic acid residues in BBE-like enzymes from Morus alba promote a function shift from oxidative cyclization to dehydrogenation
Nianxin Guo, Jun Gu, Qingyang Zhou, Fang Liu, Haoran Dong, Qi Ding, Qixuan Wang, Dongshan Wu, Jun Yang, Junping Fan, Lei Gao*, Kendall N. Houk*, and Xiaoguang Lei*
PNAS, 2025, 122 (34) e2504346122. DOI: 10.1073/pnas.2504346122
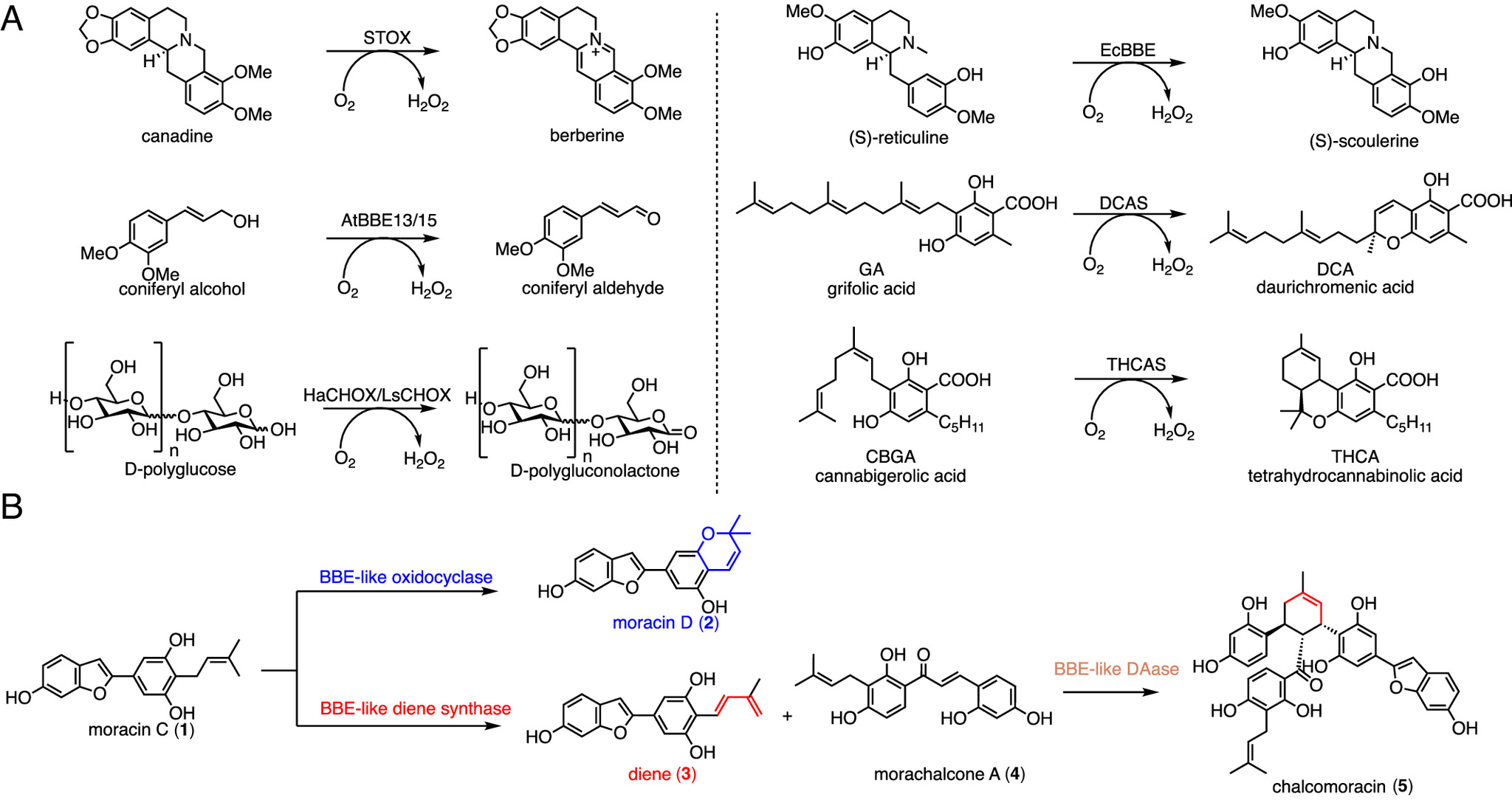
Berberine bridge enzyme (BBE)-like enzymes catalyze various oxidative cyclization and dehydrogenation reactions in natural product biosynthesis, but the molecular mechanism underlying the selectivity remains unknown. Here, we elucidated the catalytic mechanism of BBE-like oxidases from Morus alba involved in the oxidative cyclization and dehydrogenation of moracin C. X-ray crystal structures of a functionally promiscuous flavin adenine dinucleotide (FAD)–bound oxidase, MaDS1, with and without an oxidative dehydrogenation product were determined at 2.03 Å and 2.21 Å resolution, respectively. Structure-guided mutagenesis and sequence analysis have identified a conserved aspartic acid that directs the reaction toward the oxidative dehydrogenation pathway. A combination of density functional theory (DFT) calculations and molecular dynamics (MD) simulations has revealed that aspartic acid acts as the catalytic base to deprotonate the carbon-cation intermediate to generate the dehydrogenated product, which otherwise undergoes a spontaneous 6π electrocyclization in the oxidative cyclization pathway to furnish the 2H-benzopyran product.