Radiopharmaceuticals, Radiochemical Biology and Radiocatalysis
Research in the Liu group focuses on the development of novel radiochemistry for Positron Emission Tomography (PET) and radiotherapy. We seek to discover molecular structure and activity that can contribute to interdisciplinary solutions for long-standing challenges in clinics. Our research interests and recent publications are listed as below:
1) Bench-to-Bed translation of radiopharmaceuticals for cancer theranostics (e.g. PET imaging guided boron neutron capture therapy) (Eur J Nuc Med Mol Imaging, 2021, DOI: 10.1007/s00259-021-05212-7; Theranostics, 2021, 11, 304-315);

2) New in vivo chemistry that enables PET imaging probes for localized protein activation and drug release (Nature, 2020, 579, 421–426; J Am Chem Soc, 2021, 143, 2250–2255, Angew. Chem. Int. Ed., 2021, DOI: 10.1002/anie.202106526);
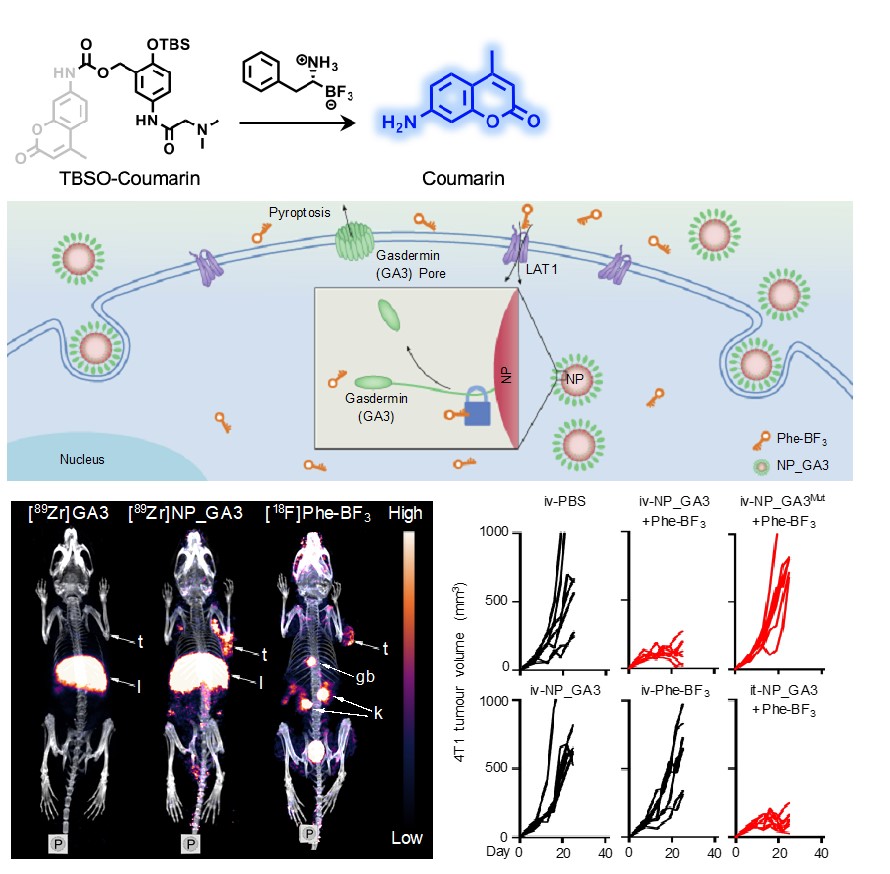
3) Radiation-activation cleavage chemistry in vivo (Angew Chem Int Ed, 2020, 132, 21730-21736);

4) Cyclotron-based isotope production and isolation, including F-18, Mn-52, Y-86 (First report in China), Zr-89, In-111, Ac-225 (First report in China) and etc.
