|
Angstrom Molecular RulerWe have been focused on developing mode specific vibrational energy transfer as a molecular ruler at the angstrom scale. The project originates from the un-satisfaction of popular methods of measuring intermolecular interactions. FRET requires structural perturbative labeling. NMR’s temporal resolution is too low. The advantages of vibrations are obvious: chemical bonds are themselves chromophores and molecular vibrations occur at the femtosecond time scale. The disadvantages of vibrations are also salient: vibrational lifetimes are short and the transition dipole moments are small. It is even worse. It is not clear at all how vibrational energy transfers from one mode to another.As to develop this approach into a useful methodology, we started from building new experimental tools, designing model systems and developing theoretical models to answer the following fundamental questions: (1) What factors determine vibrational energy flowing from one mode to the other? (2) Can the correlation between these factors and vibrational energy transfer kinetics be quantitatively described? (3) Are these factors quantitatively correlated to the molecular structural parameters, e.g. distances, transition dipole moments and refractive index? (4) Can a master analytical equation be derived to deduce molecular distances based on kinetic measurements? Our ongoing research has answered some of these questions1-4, and been heading for comprehensive understanding of the whole story.As the first demonstration of this angstrom molecular ruler, the vibrational energy transfer method was successfully applied to reveal the existence and structures of ion clusters in strong electrolyte aqueous solutions. The surprising finding opens many possible avenues for this new method, since batteries, solar cells, and biology are all associated with electrolyte solutions. We are pursuing some of these applications.
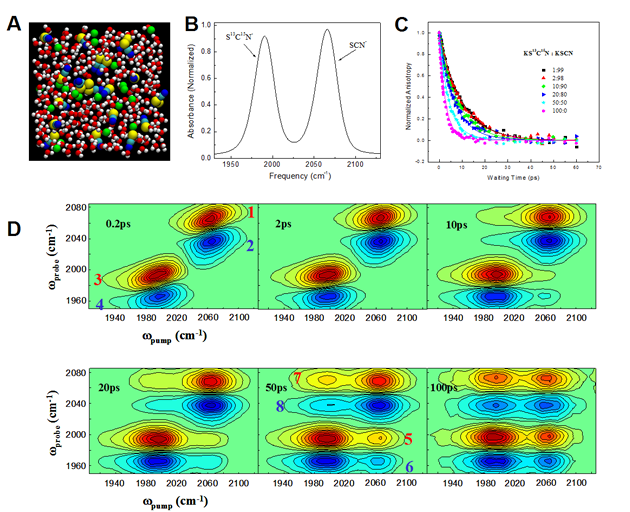
(A) A snapshot of a 1.8M KSCN aqueous solution from a molecular dynamics simulation. O (red), H (white), C (light blue), N (deep blue), K (green) and S (yellow). An ion cluster is visible at the center of the picture. Some water molecules are removed from the original file to better display the cluster structure. (B) FT-IR absorption spectra of the CN and 13C15N stretches of SCN-and S13C15N- of a 10M 1:1 KSCN/S13C15N aqueous solution (solution C). (C) The anisotropy decay data (dots) and calculations of the 13C15N stretch of S13C15N- in 10M salt aqueous solutions with different KS13C15N/KSCN ratios. Adjusting the KS13C15N/KSCN ratio changes the number of resonance energy acceptors for the excited S13C15N- donor. (D) The time dependence of the 2D IR spectrum of solution C. AsTw increases, the off-diagonal peaks grow in because of energy exchange between SCN- and S13C15N-. Only clustered anions can exchange energy. Water separated anions are too far away to vibrationally communicate with each other. From the resonant and nonresonant vibrational energy transfer measurements, the concentration, the size of the clusters and the anion distances inside the clusters are manifested.
Publications
Bian, H. T.; Li, J. B.; Wen, X. W.; Zheng, J. R. Mode-specific intermolecular vibrational energy transfer. I. Phenyl selenocyanate and deuterated chloroform mixture, J. Chem. Phys. 2010, 132, 184505.
Bian, H. T.; Wen, X. W.; Li, J. B.; Zheng, J. R. Mode-specific intermolecular vibrational energy transfer. II. Deuterated water and potassium selenocyanate mixture, J. Chem. Phys. 2010, 133, 034505.
Bian, H. T.; Chen, H. L.; Li, J. B.; Wen, X. W.; Zheng, J. R. Nonresonant and Resonant Mode-Specific Intermolecular Vibrational Energy Transfers in Electrolyte Aqueous Solutions. J. Phys. Chem. A, 2011, 115, 11657.
Bian, H. T.; Wen, X. W.; Li, J. B.; Chen, H. L.; Han, S.; Sun, X. Q.; Song, J.; Zhuang, W.; Zheng, J. R. Ion clustering in aqueous solutions probed with vibrational energy transfer, Proc. Nat. Acad. Sci. 2011, 108,
|