Home / Group News / Group News & Announcements
Two New Papers on Org. Lett. has been published online
Two of our adjacent new papers regarding Synthetic Progress toward Azadirachtins has been published online (Article One, Article Two). It's completed by Ceheng Tan, Hang Shi, Wei Chen, Ceheng Tan, Weibin Zhang, Xinpeng Mu, Zichun Zhang, Qi Chen and Rong Long instructed by Dr. Jianxian Gong, Dr. Tuoping Luo, and Prof. Zhen Yang.
Left wing:
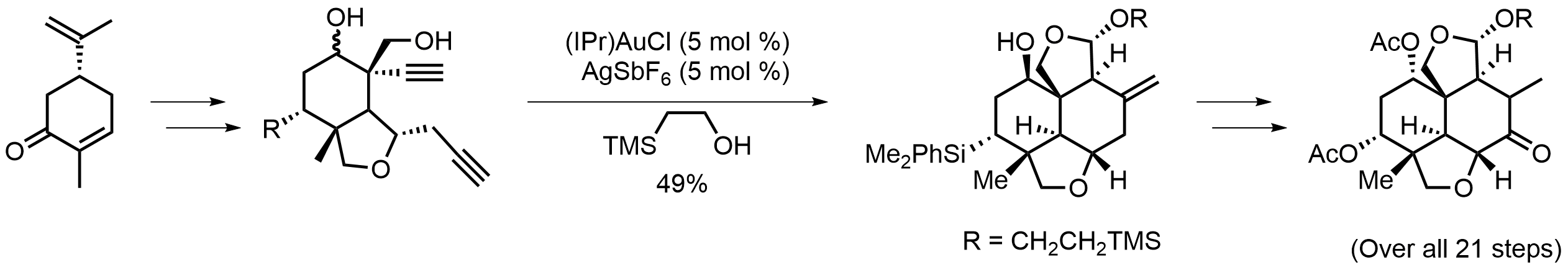
Right wing:

For the left part of the molecule, a highly enantio- and diastereoselective synthesis of the left-wing fragment of 11-epi-azadirachtin I characterized with the pairwise use of palladium- and gold-catalyzed cascade reactions is presented. By enlisting a sequence of stereocontrolled transformations, our 21-step route established the stereocenters of the left-wing fragment from one chiral starting material, (−)-carvone, which would significantly facilitate the synthetic studies of the azadirachtin-type limonoids.
And for the right part, a stereoselective three-component coupling reaction of allylzinc bromide, silyl glyoxylate, and a β-lactone has been developed. This has been successfully applied to the enantio- and diastereoselective synthesis of the fully functionalized furopyran moiety of azadirachtins.







