Quotes
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
Research Projects
Collaborations
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
Publications
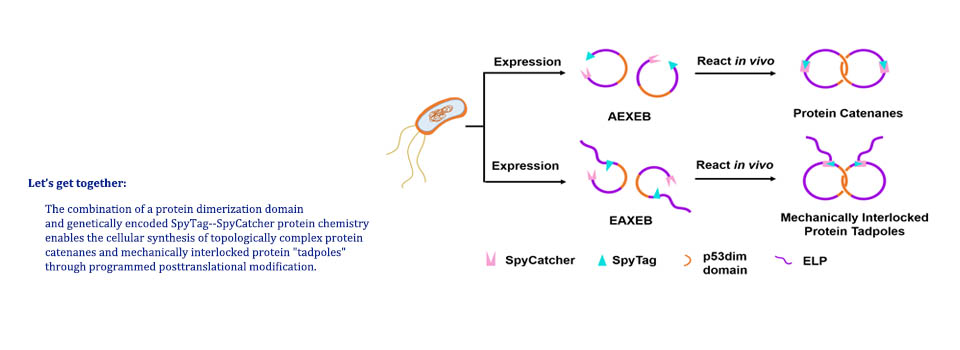
Zhang, N.;# Liu, J.;# Liu, Y.; Wu, W.-H.; Fang, J.; Da, X.-D.; Wang, S.;* Zhang, W.-B.* NMR Spectroscopic Studies Reveal the Critical Role of the Isopeptide Bond in Forming the Otherwise Unstable SpyTag−SpyCatcher Mutant Complexes, Biochemistry 2020, 59, 24, 2226–2236.
Abstract: The interplay between protein folding and chemical reaction has been an intriguing subject. In this contribution, we report the study of SpyTag and SpyCatcher reactive mutants using a combination of sodium dodecyl sulfate–polyacrylamide gel electrophoresis, liquid chromatography and mass spectrometry, circular dichroism, and NMR spectroscopy. It was found that the wild-type SpyCatcher is well-folded in solution and docks with SpyTag to form an intermediate that promotes isopeptide bond formation. By contrast, the double mutant SpyCatcherVA is disordered in solution yet remains reactive toward SpyTag, forming a well-folded covalent complex. Control experiments using the catalytically inactive mutants further reveal the critical role of the isopeptide bond in stabilizing the otherwise loose SpyTag–SpyCatcherVA complex, amplifying the effect of the minute sequence disparity. We believe that the synergy between protein folding and isopeptide bonding is an effective way to enhance protein stability and engineer protein–protein interactions.