 |
|
 |
|
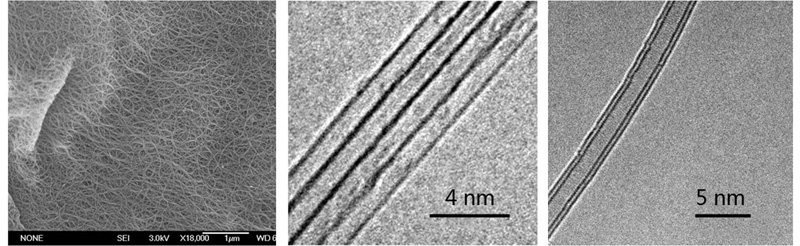
Purified SWNTs and DWNTs
|
Controllable synthesis of carbon nanotubes is a fundamental and central issue with respect to carbon nanotube research. We developed acr-discharge technique for selective and high yield syntheses of single-wall carbon nanotubes (SWNTs) and double-wall carbon nanotubes (DWNTs) (Solid State Communications 2004, 132, 219-224; Carbon, 2006, 44, 516-521). High purity SWNTs and DWNTs were obtained after purification of the original products. As a consequence of strong van der Waals force between adjacent carbon nanotubes, pristine carbon nanotubes are generally insoluble in common solvents, which hampers the application of carbon nanotubes. Covalent and noncovalent modification are effective for producing soluble or dispersible carbon nanotubes. Modified carbon nanotubes have potential applications in the fields of electronic device, biomedicine, etc.
|
Structural model of metallofullerenes based on C82 isomers;Separation of metallofullerenes with HPLC
|
Endohedral metallofullerene is a kind of fullerene derivative that is encapsulating metal atom(s) inside the carbon cage. Many fascinating properties of metallofullerenes have been elucidated in recent years. We explored the preparation of metallofullerenes with arc-discharge technique and improved the yield of metallofullerenes by an order of magnitude compared with previous methods. With this technique we prepared a series of metallofullerenes and investigated the structure, physical and chemical properties of the metallofullerenes (Chemistry of Materials 2004, 16, 2959; Chemical Physics Letters 2005, 409, 192; Carbon 2006, 44, 475; Chemistry-A European Journal 2006, 12, 562; Chemical Physics Letters 2006, 419, 44; Chemistry-an Asian Journal 2009, 4, 1703; Nanoscale 2012, 4, 6876; Chemistry-A European Journal 2012, 18, 14246; Nanoscale 2013, 5, 10409; Journal of the American Chemical Society 2013, 135, 4187).
|
|
|
Graphenes
|
 |
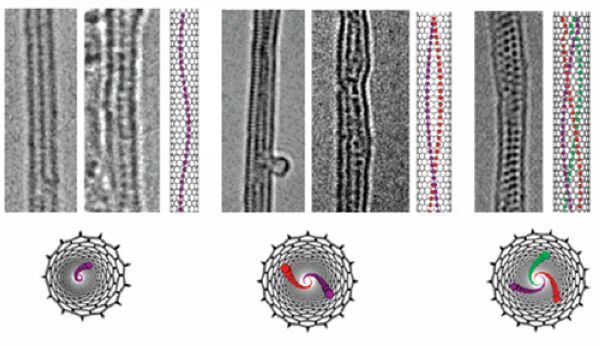
Helical iodine chains inside SWNTs |
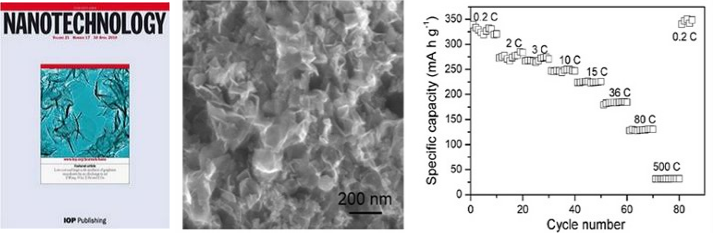
Cover of NANOTECHNOLOGY;SEM image of graphene nanosheets;Performance of graphene as anode materials of lithium-ion batteries |
 |
 |
Graphene is two dimensional carbon nanomaterial with extraordinary electronic and mechanical properties. A low-cost and scalable method to synthesize high-quality graphene is highly sought after. We developed arc-discharge technique for producing graphene nanosheets in gram scale (Carbon 2010, 48, 255-259; Nanotechnology 2010, 21, 175602). The graphene nanosheets shows excellent performance as anode materials of lithium-ion batteries (Nano Research 2010, 3, 748-756). Graphene-based composites are promising materials for catalysis and energy field (Journal of Nanoscience and Nanotechnology 2010, 10, 6690-6693; Journal of Nanoscience and Nanotechnology 2010, 10, 6748-6751).
|
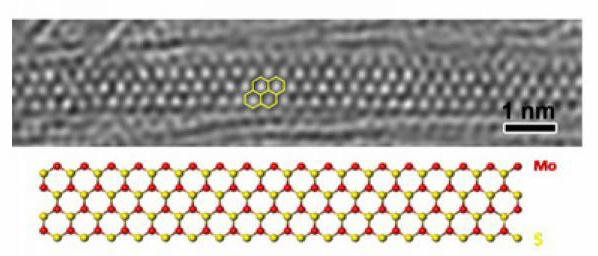
MoS2 nanoribbon inside SWNT |
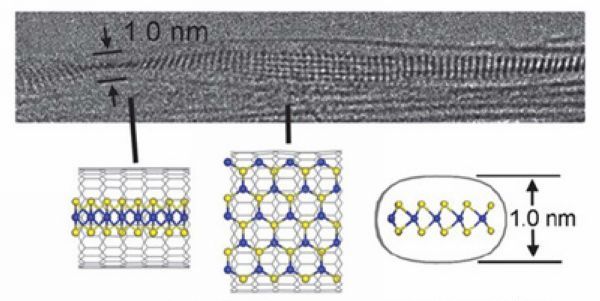
Twisted WS2 nanoribbon inside SWNT |
|
Due to the hollow structure of carbon nanotubes, they could be filled with various kinds of molecules, providing opportunities for studying the properties of molecules confined in nanospace. On the one hand, the spatial confinement imparts novel and distinct properties to the encapsulated species from their counterparts in bulk. On the other hand, the properties of carbon nanotubes are modified as a result of interaction between the carbon nanotubes and the encapsulated molecules.
Our recent work focused on the filling of arc-produced SWNTs with diameters of 1.2-1.6 nm and DWNTs with diameters of 2-5 nm. The confinement effect in these carbon nanotubes is expected to be more significant than in multi-wall carbon nanotubes with larger diameters. By using SWNTs and DWNTs as templates, we fabricated a variety of novel one-dimensional inorganic and fullerene phases. For example, single, double and triple helical iodine chains were generated inside SWNTs of smaller than 1.45 nm in diameter (Nano Letters 2007, 7, 1532-1535); MoS2 and WS2 nanoribbons with small width of 1-4 nm were synthesized through chemical reactions inside SWNTs and DWNTs (Journal of the American Chemical Society 2010, 132, 13840-13847; Journal of Materials Chemistry 2011, 21, 171-180; Nature Communications 2011, 2, 213); multiple phases of C60 and C70 were observed in SWNTs and DWNTs. In all these cases, the structure of encapsulated material is highly dependent on the diameter of the carbon nanotubes. Besides the distinctive structure, the chemical behavior of the encapsulated material may also be different from the unconfined state. We observed iodine-atom-catalyzed coalescence of C60 molecules inside SWNTs by using transmission electron microscope, and thermal coalescence experiments proved that the iodine-doped C60 molecules coalesced at lower temperatures than the undoped case (Journal of the American Chemical Society 2007, 129, 8954-8955). We investigated the pyrolysis of ferrocene molecules inside carbon nanotubes and found that ferrocene molecules transformed into carbon nanotubes with diameters strictly confined by the host nanotubes (Carbon 2005, 43, 2780-2785; Chemical Communications 2007, 1092-1094). As a consequence of interaction between carbon nanotubes and encapsulated materials, the properties of carbon nanotubes are modified. On the basis of careful analysis of reversible conversion between polyiodide anions and iodine molecules inside SWNTs and concomitant electron transfer, we developed a simple route for tuning the doping level of SWNTs (Chemical Communications 2008, 3429-3431).
|
Carbon Nanohorns
|
|
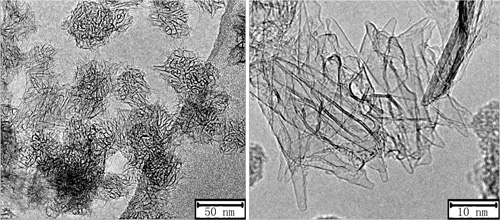
TEM image of carbon nanohorns |
Carbon nanohorn is single-layered tubular carbon nanomaterial with a horn-shape tip. Large-scale preparation of carbon nanohorns has been achieved with arc-discharge technique we developed (Carbon 2010, 48, 1580-1585). Due to its hollow structure and large specific surface area, carbon nanohorn could be used as catalyst carrier and drug delivery system.
|
|