
Research Highlights
Asymmetric Carbene-Alkyne Metathesis-mediated Cascade: Synthesis of Benzoxazine Polychiral Polyheterocycles and Discovery of a Novel Pain Blocker
Asymmetric Carbene-Alkyne Metathesis-mediated Cascade: Synthesis of Benzoxazine Polychiral Polyheterocycles and Discovery of a Novel Pain Blocker
Shuhao Liu, Haoyi Yang, Jirong Shu, Linna Wu, Yukai Li, Zhijing Zhang, Weijie Guo, Shuxian Cai, Fuyi Li, Wenjiang Liu, Shikun Jia, Song Cai, Taoda Shi, and Wenhao Hu
Angew. Chem. Int. Ed. 2024, e202401189
https://www.doi.org/10.1002/anie.202401189
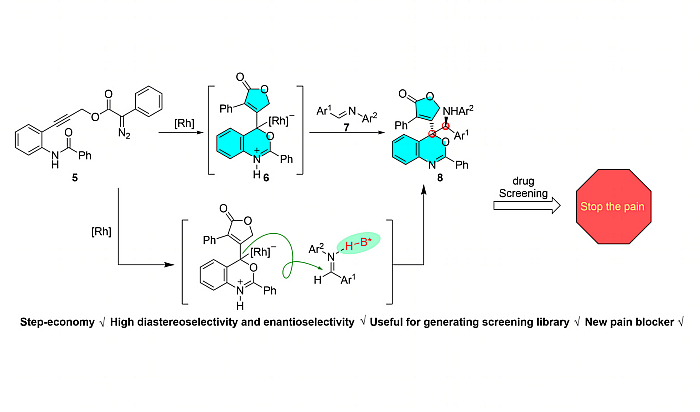
This study introduces a novel approach for synthesizing Benzoxazine-Centered Polychiral Polyheterocycles (BPCPHCs) via an innovative asymmetric carbene-alkyne metathesis-triggered cascade. Overcoming challenges associated with intricate stereochemistry and multiple chiral centers, the catalytic asymmetric Carbene Alkyne Metathesis-mediated Cascade (CAMC) is employed using dirhodium catalyst/Brønsted acid co-catalysis, ensuring precise stereo control as validated by X-ray crystallography. Systematic substrate scope evaluation establishes exceptional diastereo- and enantioselectivities, creating a unique library of BPCPHCs. Pharmacological exploration identifies twelve BPCPHCs as potent Nav ion channel blockers, notably compound 8g. In vivo studies demonstrate that intrathecal injection of 8g effectively reverses mechanical hyperalgesia associated with chemotherapy-induced peripheral neuropathy (CIPN), suggesting a promising therapeutic avenue. Electrophysiological investigations unveil the inhibitory effects of 8g on Nav1.7 currents. Molecular docking, dynamics simulations and surface plasmon resonance (SPR) assay provide insights into the stable complex formation and favorable binding free energy of 8g with C5aR1. This research represents a significant advancement in asymmetric CAMC for BPCPHCs and unveils BPCPHC 8g as a promising, uniquely acting pain blocker, establishing a C5aR1-Nav1.7 connection in the context of CIPN.

