
Research Highlights
A Copper-Masked Monosubstituted Carbene as a General Transmetalating Agent toward Stable Carbene Complexes
A Copper-Masked Monosubstituted Carbene as a General Transmetalating Agent toward Stable Carbene Complexes
María Álvarez, Francisco Villalba, Martina Casciotti, Francisco Molina, Giuseppe Sciortino, Agustí Lledós, Ana C. Albéniz, Tomás R. Belderrain, and Pedro J. Pérez
Chem 2024, 10, 1–17.
https://www.doi.org/10.1016/j.chempr.2024.03.009
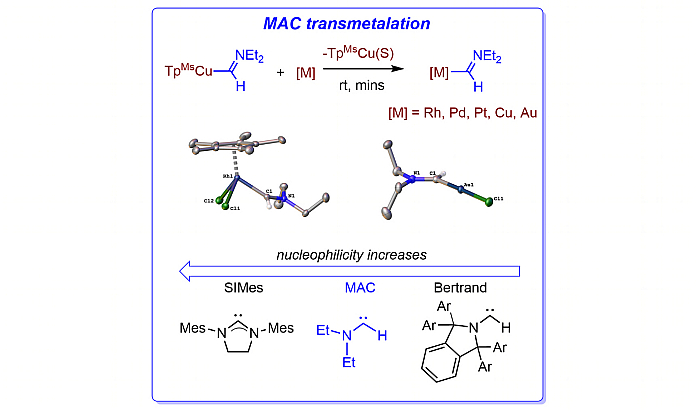
Metal complexes bearing carbene ligands LnM=CR1R2 are relevant intermediates in catalytic transformations. The stability of isolated carbene complexes decreases for the case of monosubstituted carbenes (R1 = H). We have discovered that the copper complex [TpMsCu(C(H)NEt2)] (1) readily transfers the aminocarbene :C(H)NEt2 to other complexes of metals such as rhodium, iridium, palladium, platinum, copper or gold, leading to complexes bearing the M-C(H)NEt2 moiety. Experimental data allows proposing that this monosubstituted aminocarbene (MAC) ligand displays a nucleophilicity similar to NHC ligands bearing bulky substituents but displaying lower volume. These features allow unprecedented reactivity patterns such as the generation of the first stable monosubstituted carbene adduct of Rh2(OAc)4, a well-known catalyst for carbene transfer reactions. DFT studies carried out on the transfer of the MAC ligand from [TpMsCu(C(H)NEt2)] to trans-[PdCl2(NCMe)(PPh3)] have allowed ascertaining that the transmetalation process involves several intermediates in which the TpMs ligand shifts between k2 and k3 coordination modes.

