最新热点
联系我们
张文彬课题组
地址:北京市海淀区成府路202号
北京大学化学与分子工程学院
邮编:100871
电话:010-62766876
电邮:wenbin@pku.edu.cn

请扫以上二维码关注我们课题组的公众号。
我们将定期推送组会每周精读和泛读文献介绍以及课题组的最近新闻!
--------------------------------------------
最新成果
Zhang, F.; Liu, Y.; Shao, Y.; Zhang, W.-B.* Active Template Synthesis of Protein [n]Catenanes Using Engineered Peptide–Peptide Ligation Tools. CCS Chem. 2023, DOI: 10.31635/ccschem.023.202302762. https://doi.org/10.31635/ccschem.023.202302762
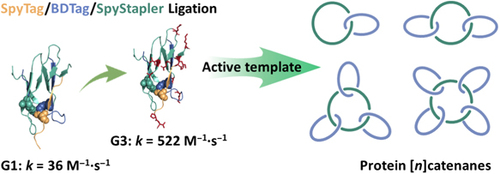
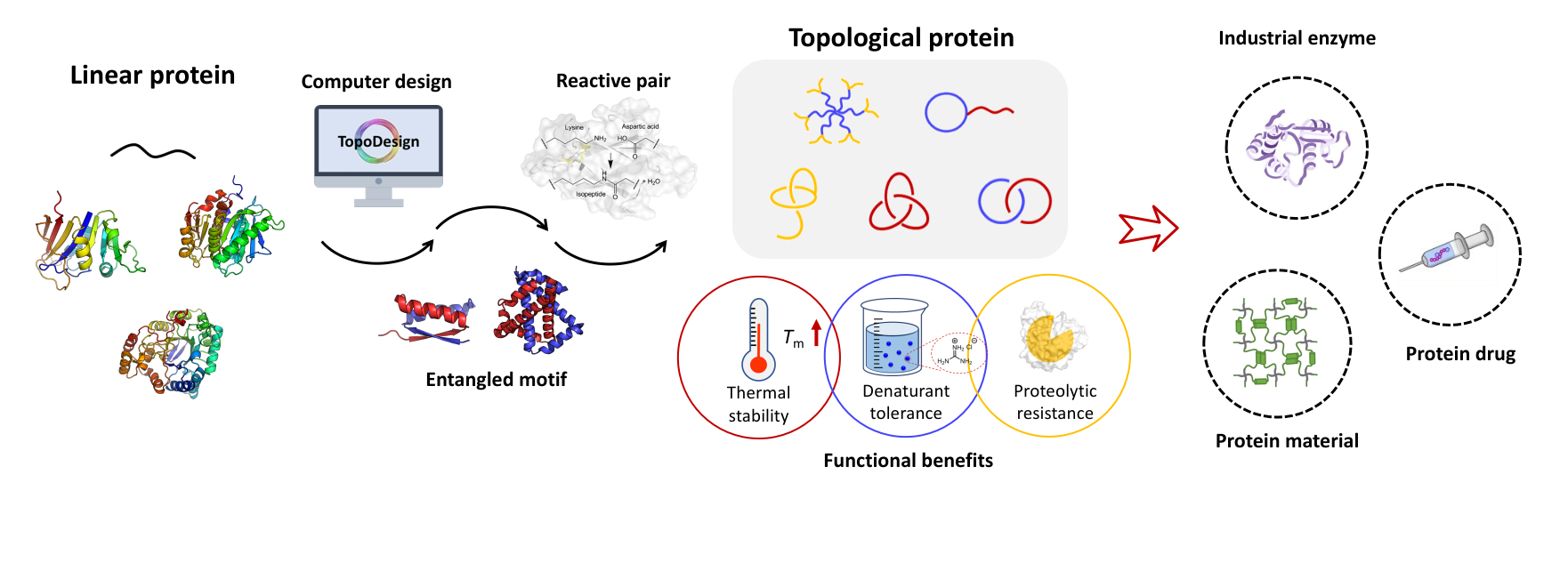
The expansion of protein topological diversity requires new and efficient synthetic tools. Herein, we report the second and third generations of the SpyStapler-mediated SpyTag/BDTag ligation system for the efficient synthesis of 4-arm star proteins and the repurposing of the third generation as an active template to enable the synthesis of higher-order protein [n]catenanes (n = 3, 4, and 5). SpyStapler003 has a higher affinity to its cognate SpyTag and BDTag reactive pairs relative to the original SpyStapler. Hence, it can overcome much more profound steric hindrance in protein ligation and improve the efficiency of the resulting active template tool to facilitate the construction of radial protein [n]catenanes. Various proteins of interest, such as dihydrofolate reductase and the nanobody KN035, can be modularly incorporated into the [n]catenanes with intact activity. Combination of passive and active template strategies gives rise to linear protein [4]catenanes, which further expands the current topological diversity. Moreover, higher-order protein catenation not only leads to enhanced thermal stability and proteolytic resistance but also higher affinity of the nanobody via multivalent effects. Our study provides tools useful for bioconjugation and new topological protein scaffolds for the multivalent display of enzymes and antibodies.