箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
研究成果
Bai, X.;# Liu, Y.;# Lee, J.; Fang, J.; Wu, W.-H.; Seo, J.; Zhang, W.-B.* Cellular synthesis of protein pretzelanes. Giant 2022, 10, 100092.
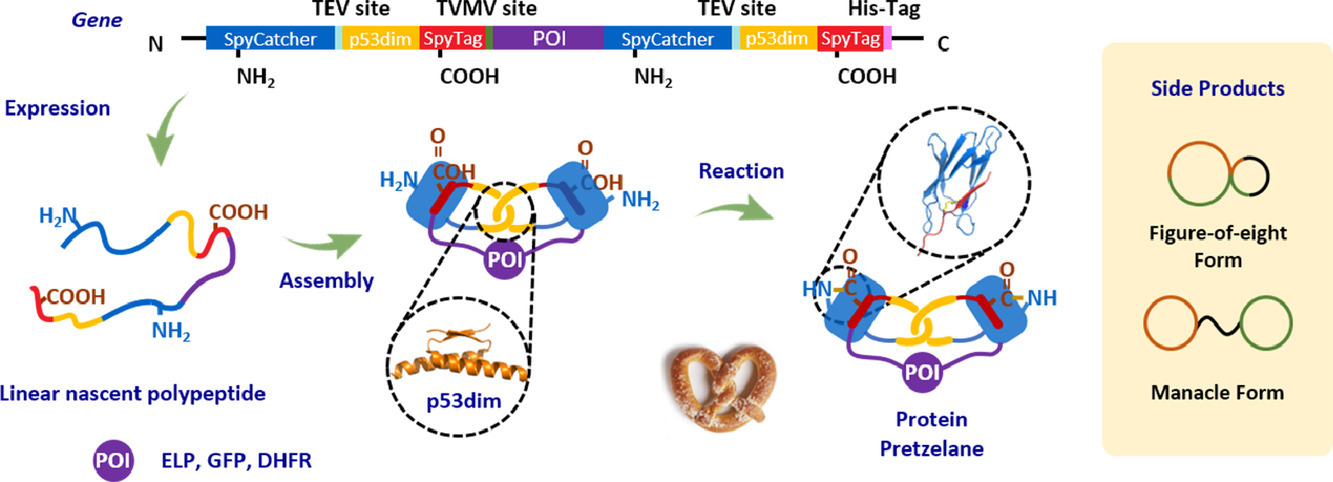
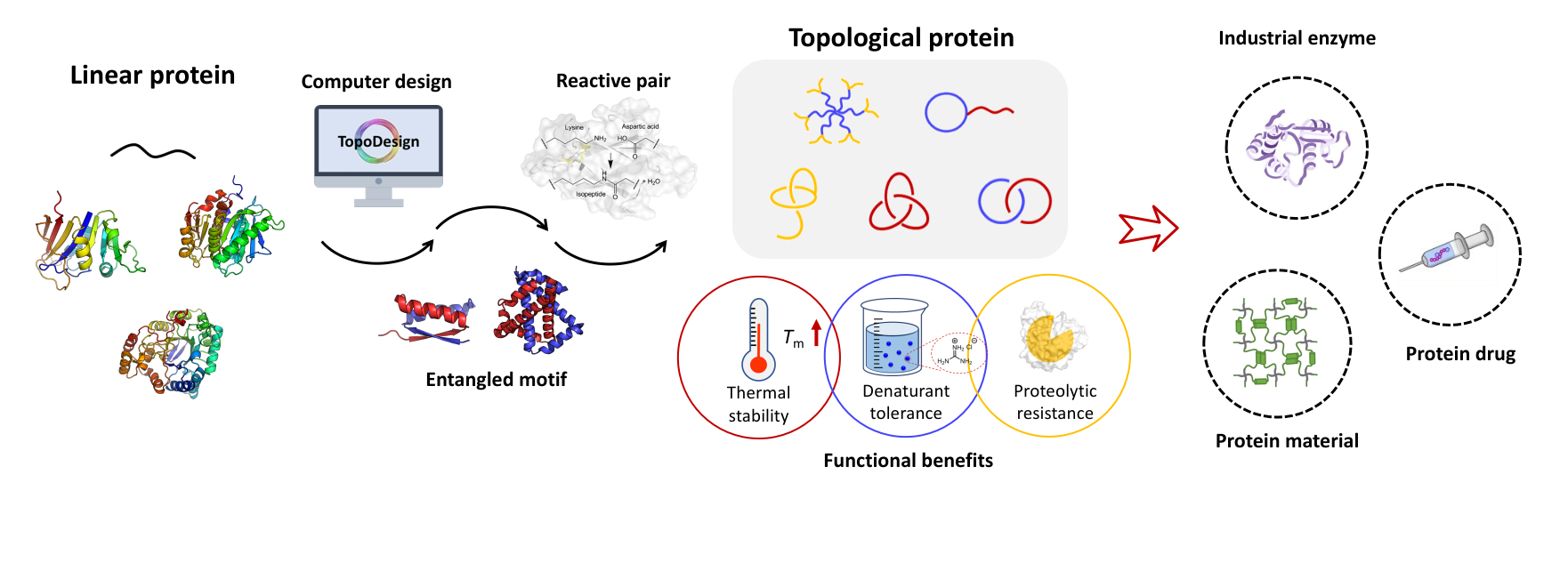
Topology has been recognized as a unique dimension in molecular engineering, yet the topological diversity remains largely untapped, especially in macromolecules. Herein, we report the molecular design, cellular synthesis, and detailed characterization of protein pretzelanes with a chemical topology of a bridged Hopf link. The synergy between the intramolecular chain entwining guided by the p53dim (X) domains and the genetically encoded side-chain coupling by SpyTag(A)-SpyCatcher(B) reaction facilitates the direct synthesis of the model protein pretzelane BXA-BXA in Escherichia coli. The approach tolerates the insertion of various proteins-of-interest, such as elastin-like protein (ELP), superfolder green fluorescent protein (GFP) and dihydrofolate reductase (DHFR), at the bridge region between two rings, giving rise to three protein pretzelanes BXA-ELP-BXA, BXA-GFP-BXA, and BXA-DHFR-BXA. Their topology has been verified by combined techniques of MALDI-TOF mass spectrometry, ion mobility-mass spectrometry, site-specific mutation, and orthogonal proteolytic digestion experiments. Not only are the fluorescent properties of GFP and the catalytic properties of DHFR fully retained, the pretzelane topology also renders BXA-DHFR-BXA more thermally resilient than the wild-type DHFR. These results expand the topological diversity of proteins and demonstrate protein stabilization as a potential functional benefit for the pretzelane topology.