箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
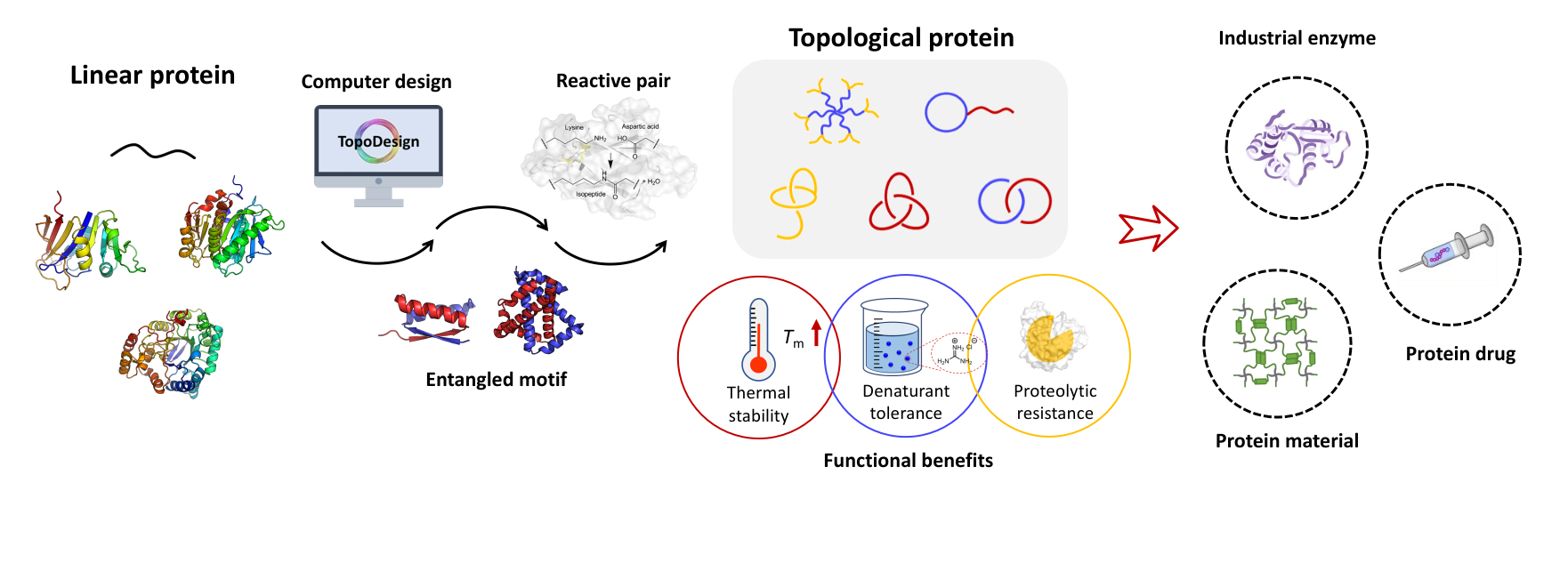
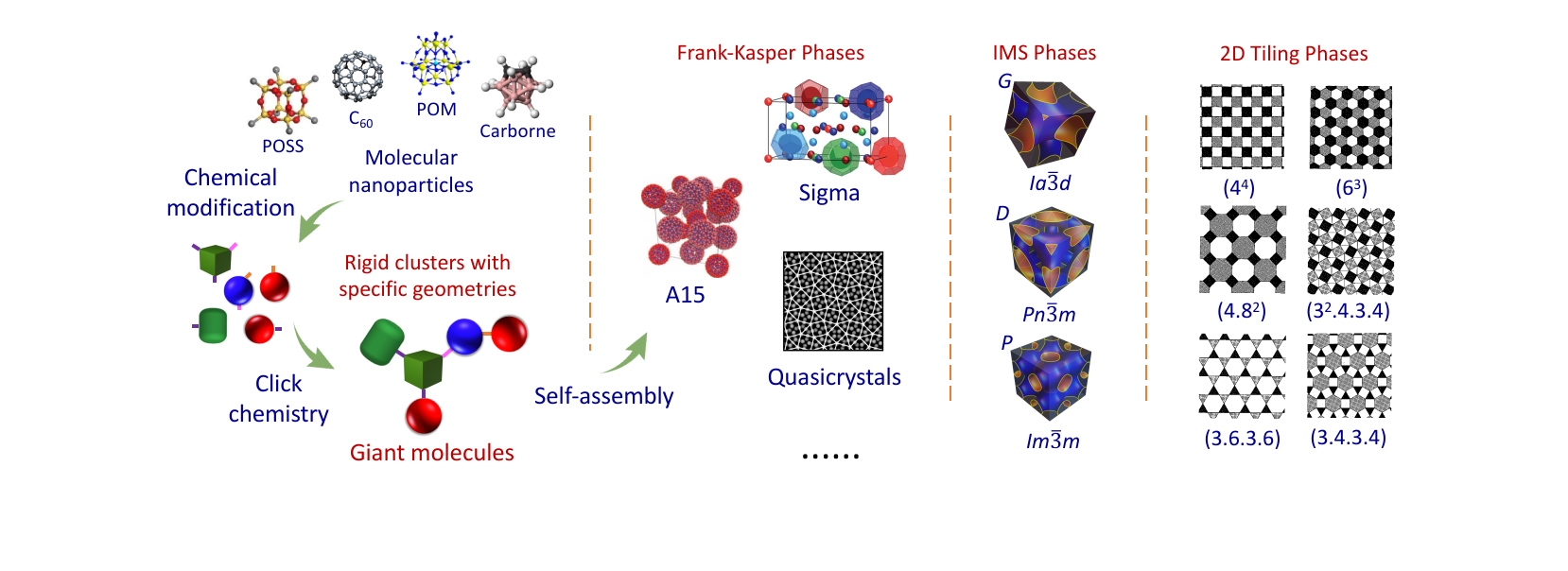
研究成果
Shao,Y.; Zhang, X.; Liang, K.; Wang, J.; Lin, Y.; Yang, S.; Zhang, W.-B.; Zhu, M.* and Sun, B.* How Does the Interplay between Bromine Substitution at Bay Area and Bulky Substituents at Imide Position Effects the Photophysical Properties of Perylene Diimides? RSC Adv., 2017,7, 16155-16162. [Link]

Abstract
This article reports a comparative study on the synthesis, self-assembly, and photophysical properties of perylene diimides (PDIs) symmetrically tethered with long alkyl chains or polyhedral oligomeric silsesquioxanes (POSS) at the imide position and/or bromo substitutions at 1,7-positions of the bay area. This series of samples include Dodecyl-PDIH-Dodecyl (1), Dodecyl-PDIBr-Dodecyl (2), POSS-PDIH-POSS (3), and POSS-PDIBr-POSS (4). In solution, the PDIs with bromine substitution at bay area (2, 4) exhibit red-shifted absorption maximum compared to those without (1, 3), which is consistent with a twisted perylene chromophore as revealed by molecular simulation. Similar bathochromatic shift was observed on the solid crystal state emission of 2 as compared to 1. However, in crystals, the emission spectrum of 4 exhibits a seemingly hypochromatic shift relative to that of 3, which could be rationalized by their packing in the crystals. The bromo substitution is believed to partially quench the fluorescence and the relatively loose packing of the twisted π-plane of 4 may not be able to confine π-plane in place, leaving multiple pathways for fluorescent quenching rather than red-shifted emission. While both 3 and 4 exhibit a unique dimer packing scheme, the dimers have quite different longitudinal offset and transverse offset of the π-plane. The longitudinal offset in dimers of 4 is so large that the naphthalene moieties in the dimer almost adopts a face-to-face arrangement and their mutual interactions are considered relatively independent. All these contribute to the less red-shifted fluorescent emission and the lower fluorescent yields in crystals of 4 relative to 3 as compared to that in solution. The study shall shed light into the complicated mutual interactions among intrinsic electronic structure, microscopic molecular packing, and the macroscopic optoelectronic properties.