Harnessing Chromium(V) Hydrazido Intermediates for N2 Functionalization to Multisubstituted Hydrazines through Ligand Migration
Xueli Wang, Yue Wu, Yixi Wang, Rong Sun, Junnian Wei*, Zhenfeng Xi*
J. Am. Chem. Soc. 2025. DOI: 10.1021/jacs.5c09083.
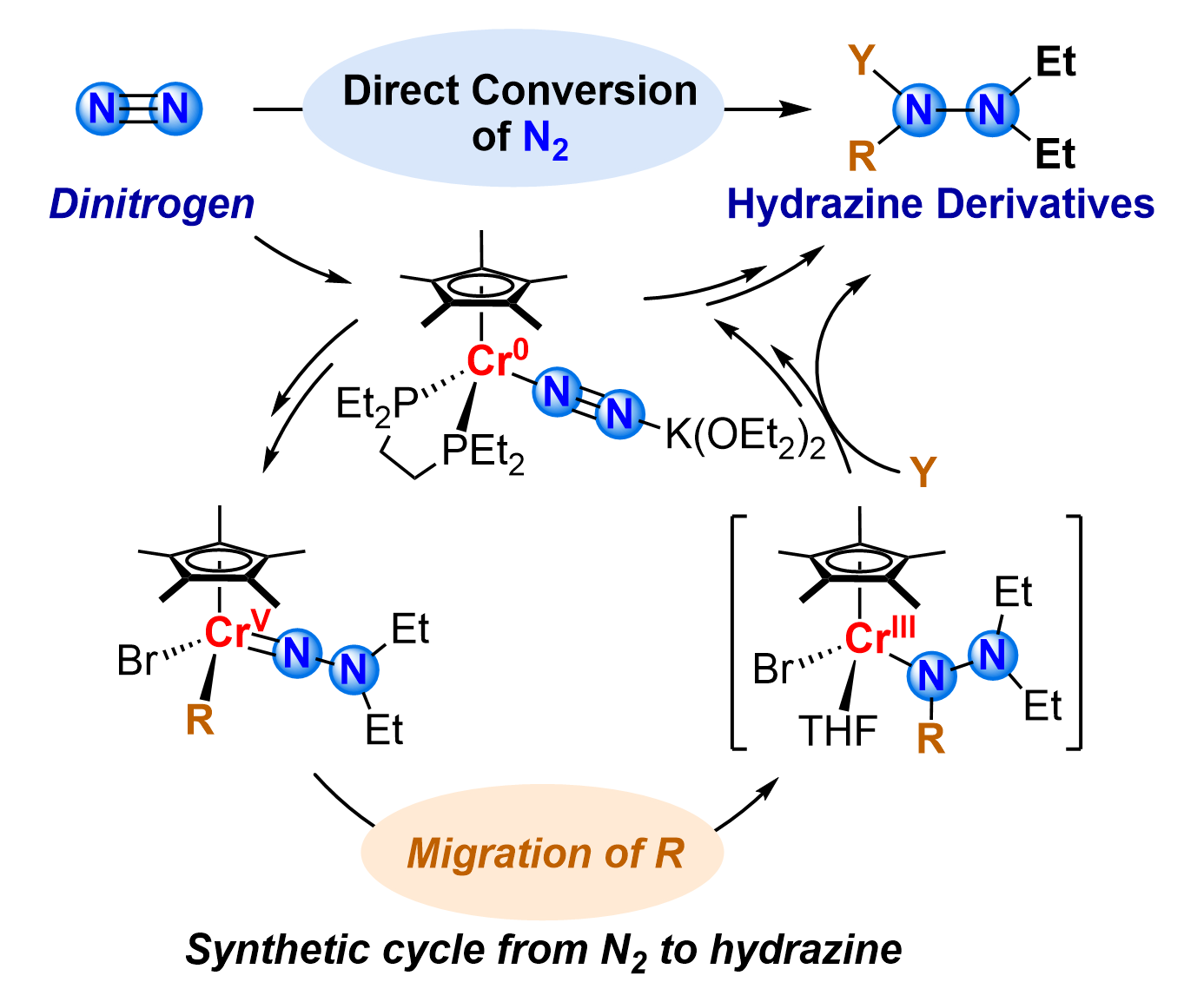
The direct conversion of N2 into N-containing organic compounds remains a fundamental challenge in synthetic chemistry. Here, we report a high-valent chromium(V)-mediated strategy for synthesizing multisubstituted hydrazine derivatives via ligand migration, representing ligand migration to Nα in a Cr-hydrazido complex. Our approach begins with the oxidation of chromium(IV) hydrazido precursor 1 by benzyl bromide, which replaces the 1,2-bis(diethylphosphino)ethane (depe) ligand with two bromide ions to generate the high-valent chromium(V) dibromide hydrazido complex 2. Sequential ligand metathesis of 2 with aryl Grignard reagents (ArMgBr, Ar = biphenyl, p-methylphenyl, o-methoxyphenyl), alkenyl or alkyl Grignard reagents (1-phenylvinylmagnesium bromide and isopropylmagnesium bromide), followed by ligand migration from the Cr center to the Nα atom and electrophilic trapping (MeOH, PhCH2Br), affords structurally diverse hydrazines (4a–c, 5a–c, and 6). Single-crystal X-ray crystallography, in situ UV–vis spectroscopy, and DFT calculations collectively reveal a ligand migration mechanism in chromium chemistry, directly forging N–C bonds from N2 without ammonia intermediates. This migration not only constructs new N–C bonds but also enables the direct construction of complex hydrazine structures directly from N2.