Unusual Electronic Structures of an Electron Transfer Series of [Cr(μ-η1:η1-N2)Cr]0/1+/2+
Gao-Xiang Wang,* Chunxiao Shan, Wang Chen, BotaoWu, Peng Zhang, Junnian Wei,* Zhenfeng Xi, Shengfa Ye*
Angew. Chem. Int. Ed., 2024. DOI: 10.1002/anie.202315386.
In dinitrogen (N2) fixation chemistry, bimetallic end-on bridging N2 complexes M(μ-η1:η1-N2)M can split N2 into terminal nitrides and hence attract great attention. To date, only 4d and 5d transition complexes, but none of 3d counterparts, could realize such a transformation. Likewise, complexes {[Cp*Cr(dmpe)]2(μ-N2)}0/1+/2+ (1-3) are incapable to cleave N2, in contrast to their Mo congeners. Remarkably, cross this series the N-N bond length of the N2 ligand and the N-N stretching frequency exhibit unprecedented nonmonotonic variations, and complexes 1 and 2 in both solid and solution states display rare thermally activated ligand-mediated two-center spin transitions (STs), distinct from discrete dinuclear spin crossovers. In-depth analyses using wave function based ab initio calculations reveal that the Cr-N2-Cr bonding in complexes 1-3 is distinguished by strong multireference character and cannot be described by solely one electron configuration or Lewis structure, and that all intriguing spectroscopic observations originate in their sophisticate multireference electronic structures. More critical is that such multireference bonding of complexes 1-3 is at least a key factor that contributes to their kinetic inertness toward splitting N2. The mechanistic understanding is then used to rationalize the disparate reactivity of related 3d M(μ-η1:η1-N2)M complexes compared to their 4d and 5d analogs.
双核金属端基桥式氮气配合物M(μ-η1:η1-N2)M是氮气活化与转化过程中的关键中间体之一。不同于单核金属氮气配合物通过多步质子耦合的电子转移反应(M-N2 → M-N=N-H → M=N-NH2 → M≡N)逐步断裂氮氮键的活化模式,双核金属氮气配合物可以直接断裂氮氮三键,生成金属氮化物(M(μ-η1:η1-N2)M → 2 M≡N)。目前,这类氮气撕裂反应的报道主要集中于4d和5d金属(Mo, W, Nb, Ta, Re等),3d金属未见报道。在均相氮气活化领域,“失败”往往才是主旋律,相较于少数“成功”案例,探明3d金属双核氮气配合物不能断裂氮气的内在原因更为关键。据此,课题组与大连化物所叶生发团队合作,对双核铬桥式氮气配合物的电子结构提出了新的见解,并阐释了其不能发生氮气撕裂反应的本质原因。
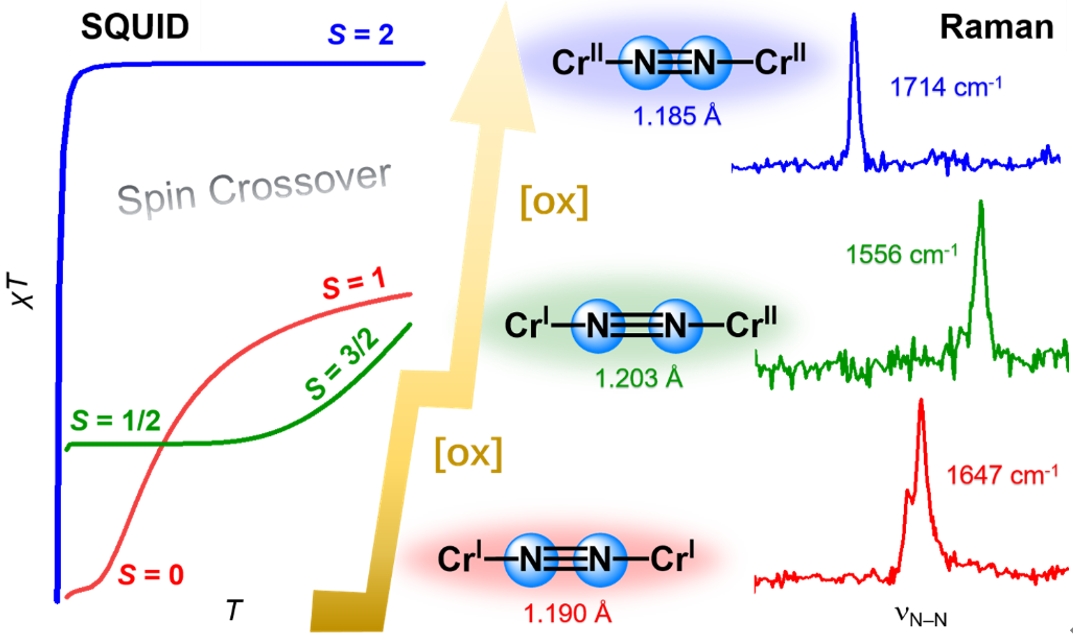
我们利用SQUID,EPR,Evans磁化率,Raman,UV-vis等一系列光谱学表征手段结合高精度的从头算方法,开展了一系列双核铬氮气配合物{[Cp*Cr(dmpe)]2(μ-N2)}0/1+/2+电子结构的系统性研究。实验结果表明双核铬氮气配合物在固态和溶液态下均存在自旋交叉(spin crossover)现象。其中,{[Cp*Cr(dmpe)]2(μ-N2)}0出现S = 0 到S = 1的自旋交叉,{[Cp*Cr(dmpe)]2(μ-N2)}1+出现S = 1/2 到S = 3/2的自旋交叉。这类双核金属配合物的自旋交叉现象极为罕见,自旋态的转变并不是单个过渡金属中心的行为,而是Cr-N2-Cr单元整体自旋态的变化。多光谱学表征结果说明这些铬氮气配合物具有很近的激发态,其电子结构具有多组态性质。CASSCF/NEVPT2的计算结果也进一步证实了双核铬氮气配合物的多组态电子结构特征。
我们认为,正是由于这种多组态的金属氮气键合模式,“稀释”了能够发生氮气撕裂的有效电子组态((1πu)4(2πg)4(3πu)2),因而氮氮键的裂解反应不能顺利发生,即该反应本身存在着较大的动力学阻碍,不仅正向反应难以进行,逆向过程同样无法发生。此外,这种多组态的性质也体现在随着逐步单电子氧化,双核铬氮气配合物氮氮键键长(1.190(3) Å → 1.203(4) Å → 1.184(4) Å)和氮氮键伸缩振动频率(1647 cm-1 → 1556 cm-1 → 1714 cm-1)的不规则变化上。几何结构的变化是电子结构发生改变的直接体现,作者因为在晶体结构上注意到了这一细微的键长变化,才决定利用各种光谱学表征手段来阐明其背后的电子结构特征。这一成果近期发表在Angewandte Chemie International Edition上,文章的第一作者是北京大学博士后王高翔。