研究室工作进展 Jun. 11th, 2019
Tetralithio Metalla-aromatics with Two Independent Perpendicular
Dilithio Aromatic Rings Spiro-fused by One Manganese Atom
Since the concept of aromaticity represents one of the most fundamental and important principles in chemistry, the search for unprecedented and exciting aromatic systems, therefore, continues to drive research in this area. The chemistry of metalla-aromatics continues to be a unique and fascinating topic both theoretically and experimentally, largely because of their unusual chemical bonding and synthetic challenges.
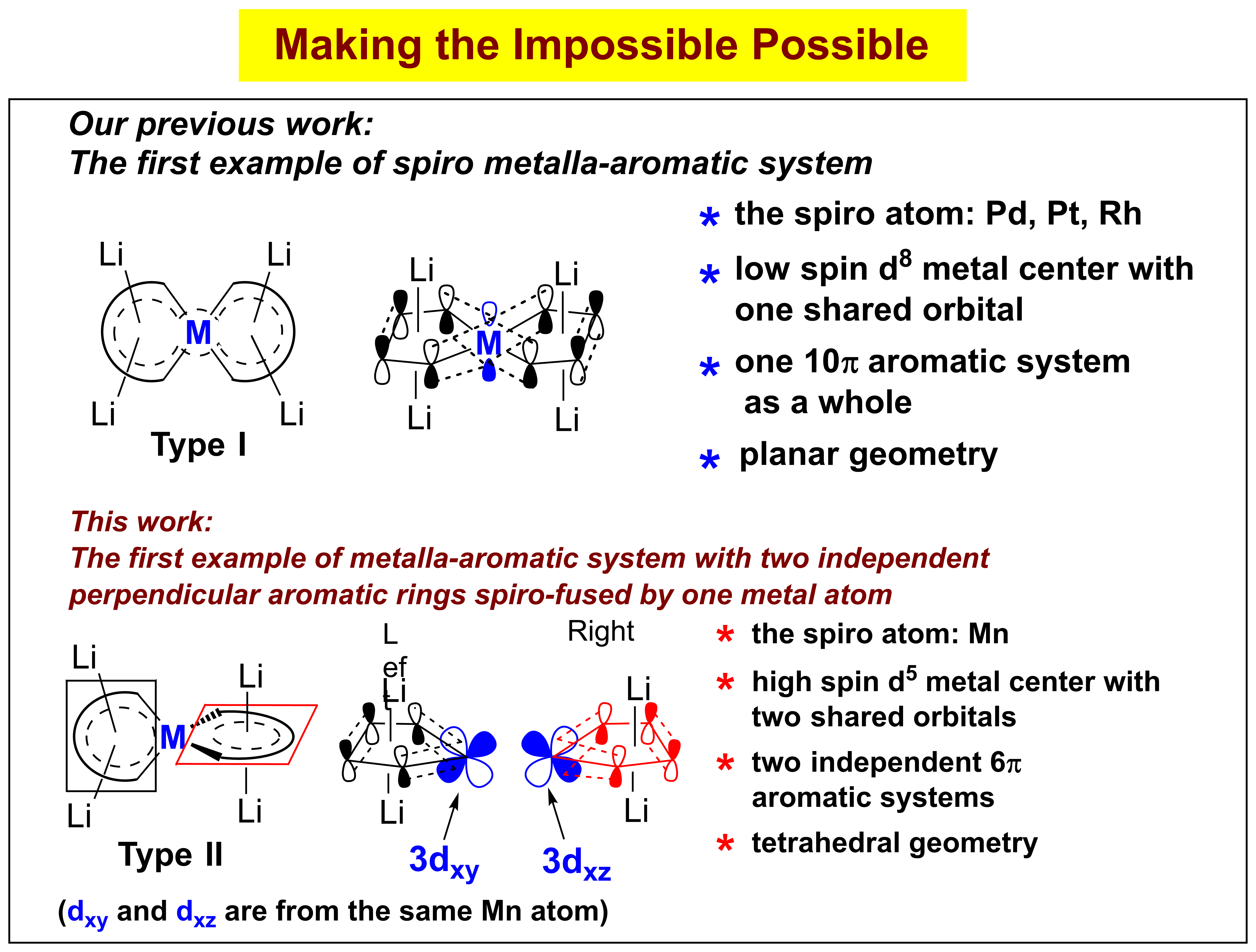
In this work, we realized a class of unprecedented aromatic structures Type II: metalla-aromatics with two independent and perpendicular aromatic rings spiro-fused by a transition-metal spiro atom, of which their corresponding organic analogues are impossible. Tetralithio spiro manganacycles Type II are readily synthesized from dilithio reagents and MnCl2 in the presence of lithium. Its aromaticity is supported by experimental measurements (X-ray structural analysis, NMR) and theoretical analyses (NICS, ACID, MOs). The spiro atom Mn uses its 3dxz and 3dxy orbitals to form the two perpendicular manganacycles, which are two independent 6π aromatic systems. Theoretical analyses reveal that the Li cations play an indispensable role in governing their geometric and electronic structures and hence their aromaticity. Therefore, this work contributes not only to enrich the concept of aromaticity, but also to deepen the understanding of the fundamental chemical bonding.
芳香性是自然科学中最基础最重要的概念之一,而芳香性体系在化学、材料科学及生命科学等领域发挥着重要作用。因此,合成新的芳香性体系是发现新功能的重要源泉,具有重要意义。螺芳香性可以定义为螺原子参与形成的芳香体系,前所未有。
螺环化合物[碳为螺原子]是有机化学中常见的环状化合物。碳原子的成键本质决定了有机螺环化合物不可能具有芳香性。但是,如果把碳螺原子换成过渡金属,则构成金属杂螺环化合物[金属离子为螺原子]。由于金属的轨道特性,金属杂螺芳香性化合物就成为可能。本研究室已经发现双锂试剂的共轭丁二烯骨架能够同两个碳负离子产生“协同效应”,使其LUMO轨道能级相对于1,3-丁二烯显著降低,从而可以接受合适的过渡金属的d电子并离域至其π*轨道(魏俊年等,Angew. Chem. Int. Ed. 2015, 54, 5999; 2015, 54, 9986; J. Am. Chem. Soc. 2016, 138, 60.)。2017年,张永亮等同学利用双锂试剂的这一独特性质并结合过渡金属配位化学,首次成功合成了螺原子为Pt, Pd, Rh的金属杂螺芳香化合物(张永亮等,J. Am. Chem. Soc. 2017, 139, 5039)。在该类芳香性体系中(上图I),过渡金属螺原子利用其一个pz轨道中的两个电子与周边八个碳原子的八个电子构成一个10π电子芳香性结构,每一个五元环都是平面的,而两个五元螺环也趋向于相互平行;但是,在本工作中(上图II),张永亮等同学合成了第二类螺芳香性化合物,该类螺芳香性化合物与第一类显著不同,其中螺原子Mn利用其3dxy和3dxz轨道分别与左右的两个四碳共轭部分构成了两个独立的五元6π电子平面芳香性结构,而两个五元螺环是相互垂直的。
那么,第三类螺芳香性化合物将以什么样的结构和成键模式问世呢?这些螺芳香性化合物又将具有什么样的反应化学和功能呢?我们拭目以待!
