研究室工作进展 Mar. 19th, 2013.
DFT Studies on the Reaction Mechanisms of 1,4-Dilithio-1,3-dienes with Nitriles
Fei Zhao, Ming Zhan, Wen-Xiong Zhang, and Zhenfeng Xi*
Organometallics 2013, DOI: 10.1021/om300869t
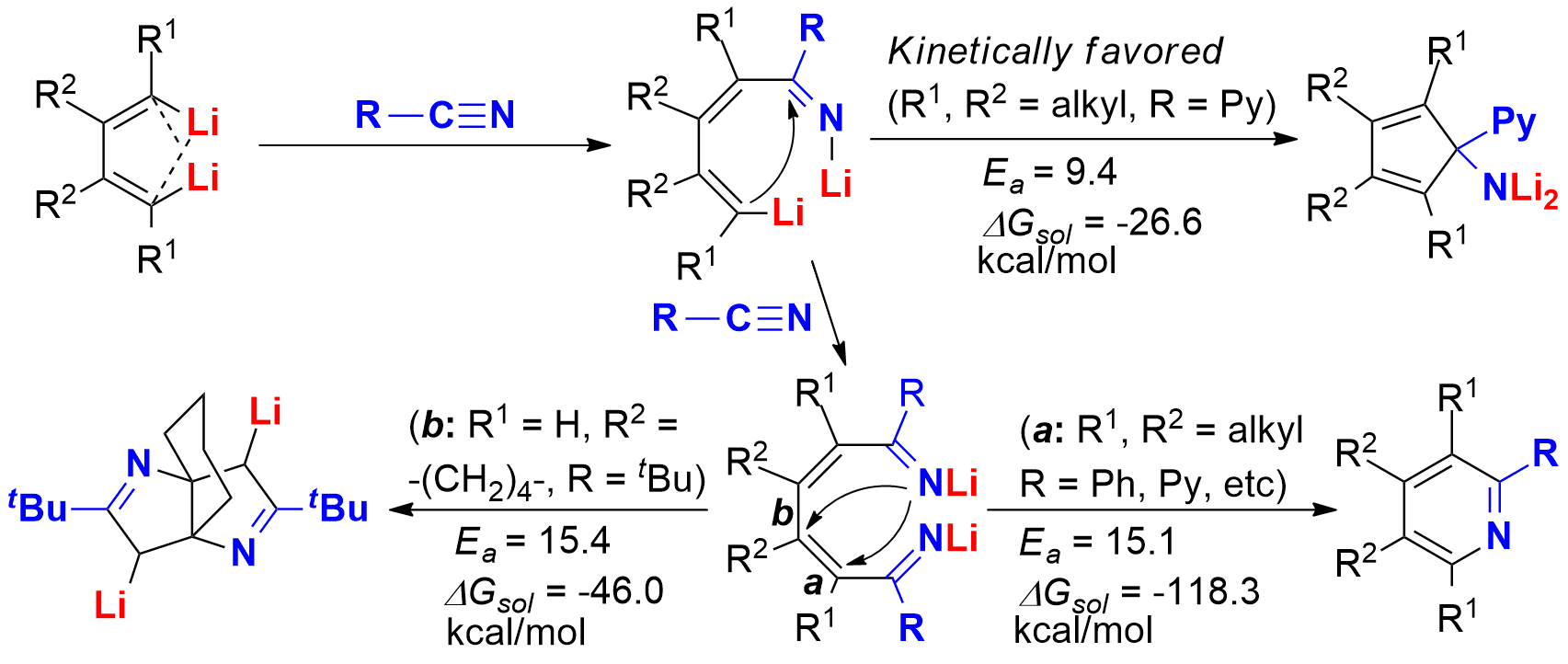
Mechanisms on the reactions of 1,4-dilithio-1,3-butadienes and nitriles are explored through both experiments and DFT calculations. The computational results suggest that the selectivity of these reaction systems is strongly affected by the structures of the substrates. As the first step of all reaction pathways, the addition intermediate of one C-Li bond to the nitrile is formed. When tetra-alkyl substituted 1,4-dilithio-1,3-dienes and 2-cyanopyridine are used, the intermediate gives the cyclopentadienyl amine product as the kinetic product because of the coordination of the pyridyl N-atom to the lithium atoms. This addition intermediate also undergoes a second nitrile insertion into the C-Li bond, giving the dilithio ketimine intermediate. When tetra-alkyl substituted 1,4-dilithio-1,3-dienes and aryl nitriles are used, the dilithio ketimine intermediate undergoes a 1,6-cyclization, generating pyridine and triazine products through thermodynamically favored pathways. When cyclic 1,4-dilithio butadiene and tertiary aliphatic nitriles are used, the dilithio ketimine intermediate undergoes two sequential 1,5-cyclization steps with a lower energy barrier, generating tricyclic D1-bipyrrolines.
背景介绍
本研究室双锂试剂与腈的反应,首次报道是在2002年(陈敬龙、宋秋玲等,JACS2002)。2007年我们报道了丁二烯基双腈与有机锂的反应(王从洋、王超等,CEJ2007),2008年我们以全文的形式报道了双锂试剂与腈的三种不同反应类型(郁楠、王从洋等,CEJ2008),2012年我们又以双锂试剂与腈的反应中间体为出发物实现了氮杂半瞬烯的合成(张韶光、魏俊年等,JACS2012)。“协同效应”以及“基于活性中间体和机理研究的合成化学”是本研究室提出并坚持的科学研究理念。对于反应机理研究,通过实验分离或者捕捉活性中间体是我们近年来一贯坚持的研究方法。但是,有些反应活性中间体的实验分离与表征十分困难。例如,在双锂试剂与腈的反应中,我们发现了三种不同的反应类型。尽管先后多名研究生历经数年尝试获得反应中间体的信息,但是一直没有得到可信的实验结果。在这种情况下,我们希望借助理论计算深入理解反应机理。因此,本研究室在2010年指派赵飞同学向余志祥教授及其课题组研究生以及其他专家学习有关理论计算知识并购置了相关理论计算设备。本文是赵飞同学也是本研究室第一篇比较系统地通过理论计算阐述反应机理的论文。该工作理论计算结果结合实验结果使我们对双锂试剂与腈的三种不同反应类型的机理有了深入和全面的了解。
