研究室工作进展 May. 17th, 2013.
Mechanistic Study on the Cleavage and Reorganization of C(sp3)-H and C=N Bonds in Carbodiimides: Synthesis of 1,2-Dihydrothiopyrimidines and 2,3-Dihydropyrimidinthiones via Four-Component Coupling
Yang Wang, Fei Zhao, Yi Zhou, Yue Chi, Zitao Wang, Wen-Xiong Zhang,* and Zhenfeng Xi
Chem. Eur. J. 2013, DOI: 10.1002/chem.201301633.
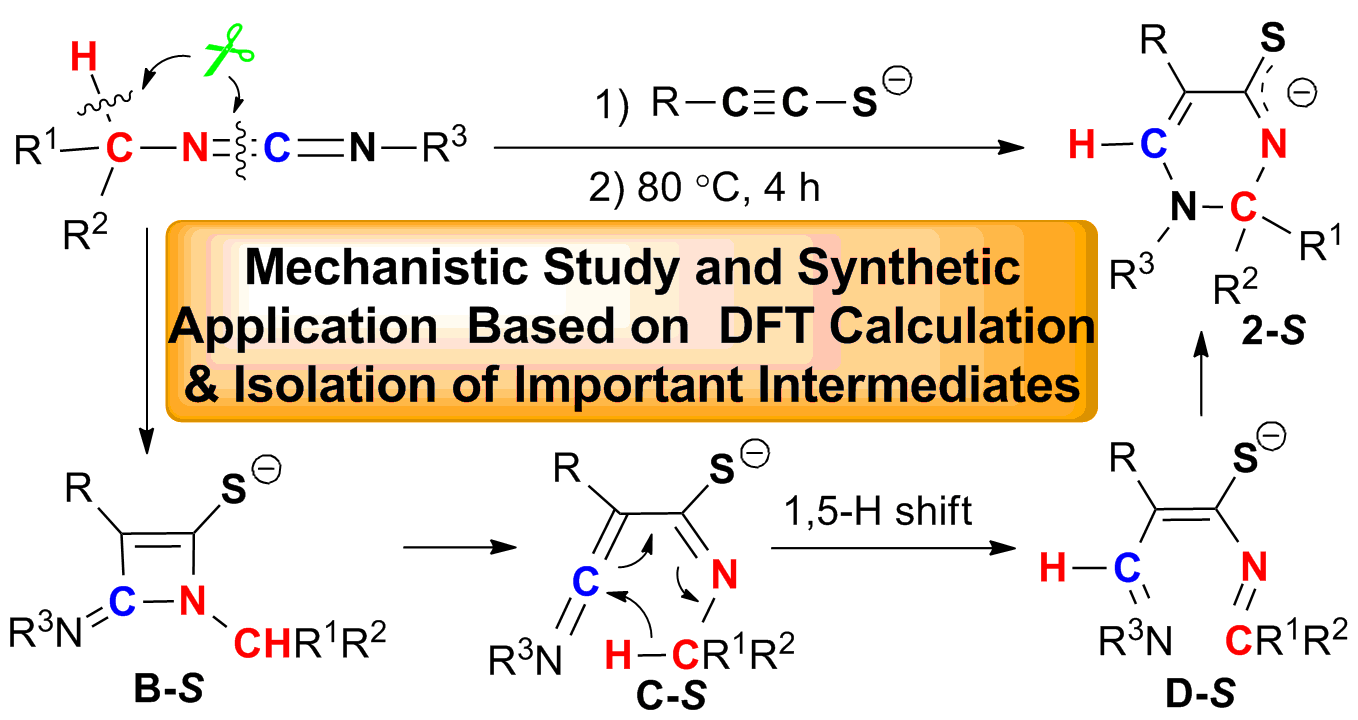
This study reveals the light of dawn on the cleavage and reorganization of C(sp3)-H and C=N bonds of carbodiimides in this three-component reaction of terminal alkynes, sulfur, and carbodiimides by the combined methods including (i) isolation and X-ray structure of six-membered-ring lithium species 2-S, (ii) trapping of the Oxygen-analogues (B-O and D-O) of both four-membered-ring intermediate B-S and ring-opening intermediate D-S, (iii) deuterium labeling studies, (iv) theoretical study. These results show that (i) this reaction rate-determining step is [2+2] cycloaddition, (ii) the C=N bond cleavage takes place before the C(sp3)-H bond cleavage, (iii) the hydrogen attached to the C6 in 2-S is from carbodiimide, (iv) three types of new aza-heterocycles, such as 1,2-dihydrothiopyrimidines, N-acyl 2,3-dihydropyrimidinthiones and 1,2-dihydropyrimidinamino acids are constructed efficiently based on 2-S. All results strongly support that the reaction undergoes through [2+2] cycloaddition/4π electrocyclic ring-opening/1,5-H shift/6π electrocyclic ring-closing as key steps. The research strategy on the synthesis, isolation and reactivity investigation of important intermediates in metal-mediated reactions not only gives the in-depth understanding of reaction mechanisms but also leads to the discovery of new synthetically useful reactions based on the important intermediates.
背景介绍
碳二亚胺(R1-N=C=N-R2),作为一类具有累积C=N双键的杂联烯类化合物,在有机合成化学与金属有机化学领域中发挥着非常重要的作用。所有报道的反应仅涉及碳二亚胺的两个反应位点:中心碳和C=N双键。自2007年以来我们一直开展金属促进或催化的碳二亚胺新反应化学及机理研究。2009年,我们发现了端炔、单质硫和碳二亚胺,在有机锂试剂的促进下,多组分偶联可制备2,3-二氢嘧啶硫酮的新反应。在这个反应中,碳二亚胺分子的一个C=N双键和一个Csp3-H键被切断。该成果为活化碳二亚胺分子中化学键和有效地利用碳二亚胺提供了一种新思路和新方法(Wen-Xiong Zhang,* Zhenfeng Xi,* et. al. J. Am. Chem. Soc. 2009, 131, 15108.)。经过近三年的努力,通过对该反应中六元环金属有机活性中间体2-S的分离和结构鉴定,氧类似物前体B-O和D-O的合成、捕获,同位素标记实验并结合计算化学等,我们揭开了该反应的机理经历: [2+2]环加成、4π-电环化开环、[1,5]-氢迁移和6π-电环化关环等关键步骤。并进一步建立了六元环中间体2-S在合成化学中的应用(Yang Wang, Fei Zhao, Yi Zhou, Yue Chi, Zitao Wang, Wen-Xiong Zhang,* and Zhenfeng Xi Chem. Eur. J. 2013, DOI: 10.1002/chem.201301633)。
