研究室工作进展 Apr 26th, 2012
Carbonylation of 1-Lithiobutadiene with Carbon Monoxide Followed by Intramolecular Acyllithiation of C=C Double Bond and Intermolecular Acylation with Acid Chloride: Scope, Applications and Mechanistic Aspects
Heng Li, Lantao Liu, Fei Zhao, Congyang Wang, Chao Wang, Qiuling Song, Wen-Xiong Zhang, and Zhenfeng Xi*
J. Org. Chem. 2012, online 20120423, DOI: 10.1021/jo300631d
The carbonylation of a 1-lithio-1,3-butadiene derivative with CO gave rise to a butadienyl acyllithio intermediate, which underwent an immediate intramolecular acyllithiation of the C=C double bond affording a lithio cyclopentadienyl enolate. The X-ray structural analysis of the enolate revealed a dimer connected with a “Li2O2” four-membered ring. Subsequent intermolecular acylation of this enolate with acid chlorides afforded β-keto-3-cyclopentenones, γ-keto-2-cyclopentenones or cyclopentadienyl ester derivatives. The stereo- and regio-selectivity of the in situ generated lithio cyclopentadienyl enolate with various acid chlorides was investigated and analyzed, showing that the formation of the above products was significantly dependent on both the substituents on the butadienyl skeleton and the bulkiness of acid chlorides.
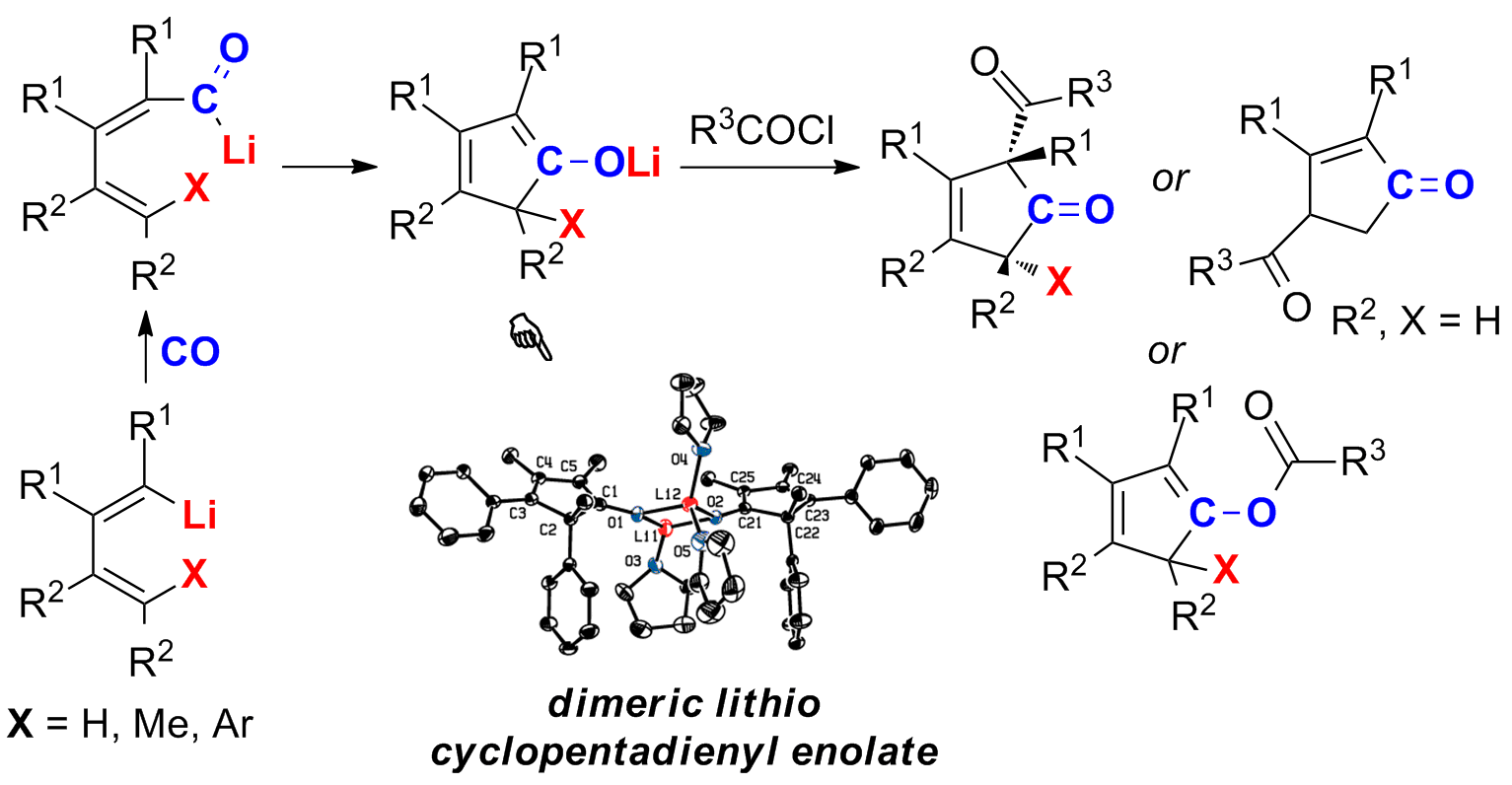
背景介绍
2002年,本研究室发现1-锂-1,3-丁二烯与一氧化碳、腈发生新颖反应,分别生成环戊烯酮衍生物和多取代吡啶衍生物 (Org. Lett. 2002, 4, 4627; J. Am. Chem. Soc. 2002, 124, 6238.)。我们推测在该反应中,有机锂部分与分子内存在的碳碳双键产生协同效应,促进分子内有机锂部分对碳碳双键的加成反应。近十年来,我们“基于机理研究和活性中间体的合成化学”研究理念,一直尝试获得该反应的中间体信息以理解该反应的机理,并进一步发展新方法。在本工作中,我们成功分离得到了1-锂-1,3-丁二烯与一氧化碳反应产生的环戊二烯基醇锂活性中间体,研究了该中间体发生酰基化时的电子效应和立体效应,合成了一系列酰基化产物。
