研究室工作进展 Feb. 12th, 2017
CuOTf-Catalyzed Selective Generation of 2-Aminopyrimidines from Carbodiimides
and Diaryliodonium Salts via Triple C(sp3)?H Functionalization
Yue Chi, Haihan Yan, Wen-Xiong Zhang,* and Zhenfeng Xi*
Chem. Eur. J. 2017, 23, 757-761 (Cover Paper).
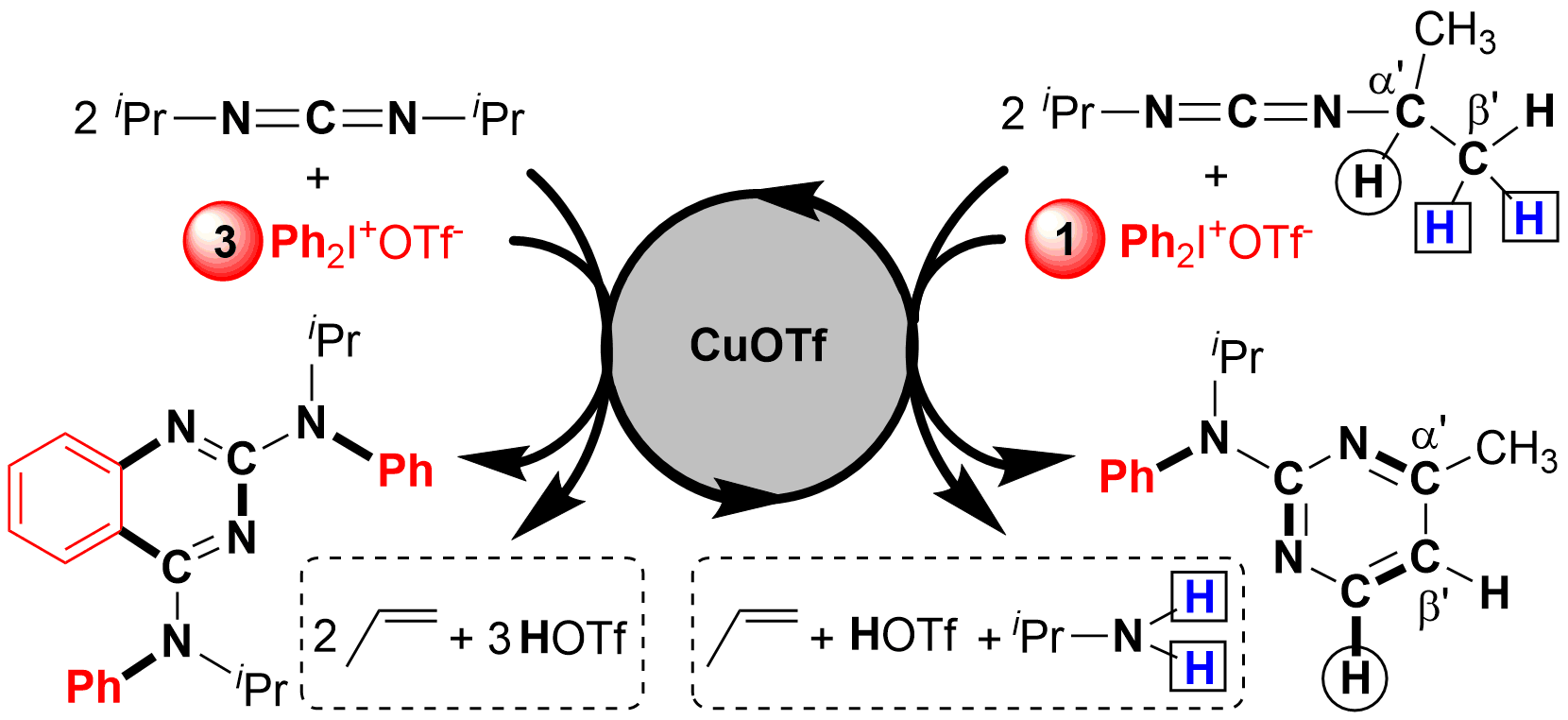
 Selective C(sp3)-H bond functionalization is an ideal and atom-economical method in organic synthesis. In this work, 2-aminopyrimidines are generated from a Cu-catalyzed reaction between carbodiimides and diaryliodonium salts via cleavage of four C(sp3)-H, one C-N, and one C=N bonds in carbodiimides. It is the first triple C(sp3)-H bond functionalization neighboring a C=N bond. The selective synthesis of 2-aminopyrimidines is controlled by the amount of diaryliodonium salts. The novel mechanism involving C-N formation/1,5-H shift/1,7-H shift/6π-electrocyclic ring-closing/aromatization is well elucidated by the detection of important intermediates and DFT calculation.
Selective C(sp3)-H bond functionalization is an ideal and atom-economical method in organic synthesis. In this work, 2-aminopyrimidines are generated from a Cu-catalyzed reaction between carbodiimides and diaryliodonium salts via cleavage of four C(sp3)-H, one C-N, and one C=N bonds in carbodiimides. It is the first triple C(sp3)-H bond functionalization neighboring a C=N bond. The selective synthesis of 2-aminopyrimidines is controlled by the amount of diaryliodonium salts. The novel mechanism involving C-N formation/1,5-H shift/1,7-H shift/6π-electrocyclic ring-closing/aromatization is well elucidated by the detection of important intermediates and DFT calculation. 