研究室工作进展 Jul. 28th, 2017
Synthesis and Structural Characterization of Butadienylcalcium-based Heavy Grignard Reagents and a Ca4[O] Inverse Crown Ether Complex
Baosheng Wei, Liang Liu, Wen-Xiong Zhang,* and Zhenfeng Xi
Angew. Chem. Int. Ed. 2017, 56, 9188–9192.
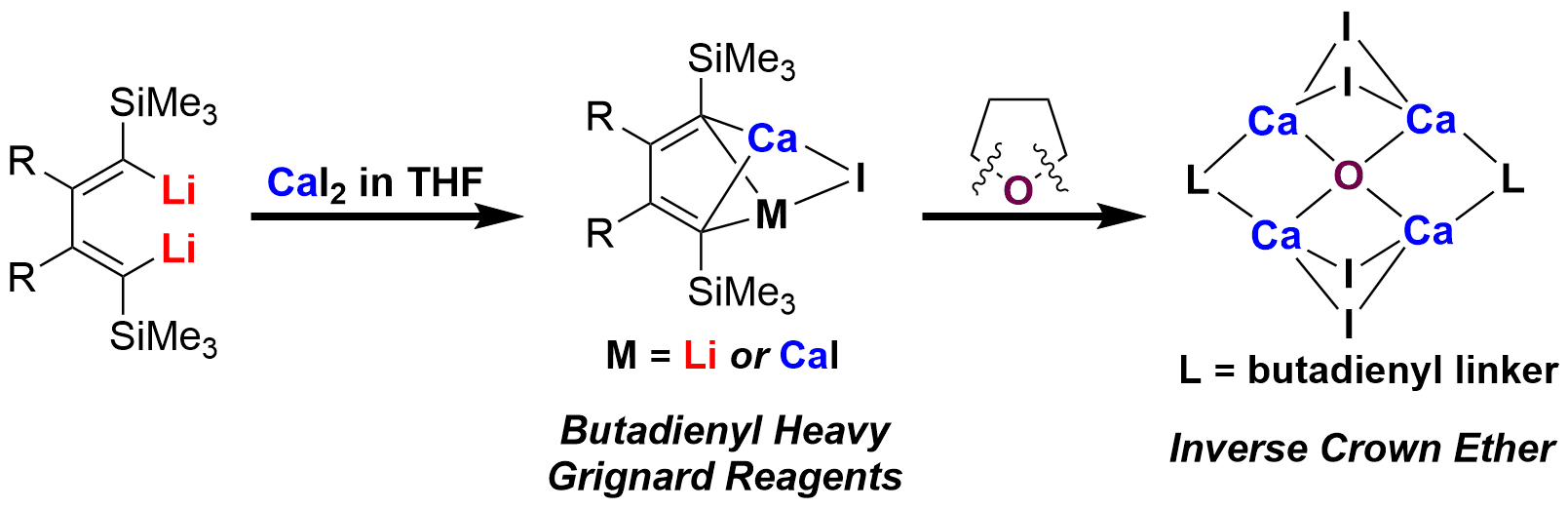
In contrast to the prosperity and great use of Grignard reagents in organometallic and synthetic chemistry, heavy Grignard reagents (RAeX, Ae = Ca, Sr, and Ba, X = halides) and the related chemistry are still in their infancy. Current study centers on the synthetic utilities of heavy Grignard reagents generated in situ, in which the reactive species mostly remain elusive because of their inaccessibility and remarkable instability. The structure elucidation of heavy Grignard reagents or corresponding reaction intermediates has been greatly strived after by chemists.
We applied the cooperative stabilizing ability of butadienyl skeletons and successfully synthesized a series of butadienylcalcium compounds, including 1-calcio-4-lithio-1,3-butadiene, 1,4-dicalcio-1,3-butadiene, and a Ca4[O] inverse crown ether complex, via the reaction between 1,4-dilithio-1,3-butadienes and calcium iodide in THF. In particular, the THF molecule was found to be degraded in an unconventional manner by virtue of the synergistic effect of dicalcium reagents, resulting in the novel inverse crown ether complex with the expanded category of bonding anions and metal cations. Single-crystal X-ray analysis of these unprecedented butadienylcalcium compounds revealed unique structural characteristics and bonding modes. This study provides a novel class of alkenyl heavy Grignard reagents and a useful synthetic strategy for otherwise unavailable reactive organometallic compounds.
亮点介绍
格氏试剂是大家熟知的金属有机试剂,与镁同族的Ca, Sr, Ba的类似物,称为重格氏试剂。由于重格氏试剂合成方法有限、稳定性差等特点,一直以来发展极为缓慢。目前的研究主要集中在重格氏试剂的原位反应性和少量稳定类型重格氏试剂的结构表征上,而对于高活性重格氏试剂的合成与表征则充满挑战。
本研究室在前期工作中发展了重格氏试剂有价值的合成应用(Angew. Chem. Int. Ed. 2013, 52, 10822–10825; Nat. Commun. 2014, 5, 4508–4517; Organometallics 2016, 35, 1458–1463.),但没有分离到体系中的反应中间体,即“丁二烯基重格氏试剂”。在该项工作中,魏保生同学首次利用转金属化反应,成功合成、分离了多种丁二烯基重格氏试剂;利用我们合成的丁二烯基双钙重格氏试剂,由于两个钙的协同反应性和钙-碳键强的离子性,它能撕裂四氢呋喃的两个C-O键,形成[Ca4O]反式冠醚。该工作为反式冠醚的合成与应用提供了一个有效途径。
