研究室工作进展 Aug. 24th, 2017
Synthesis of Dibromo- and Tetrabromo-bipyrrolines and their Corresponding 2,6-Diazasemibuvallene Derivatives
Zhe Huang, Ming Zhan, Shaoguang Zhang, Qian Luo, Wen-Xiong Zhang* and Zhenfeng Xi*
Org. Chem. Front. 2017, 4, 1785–1788.
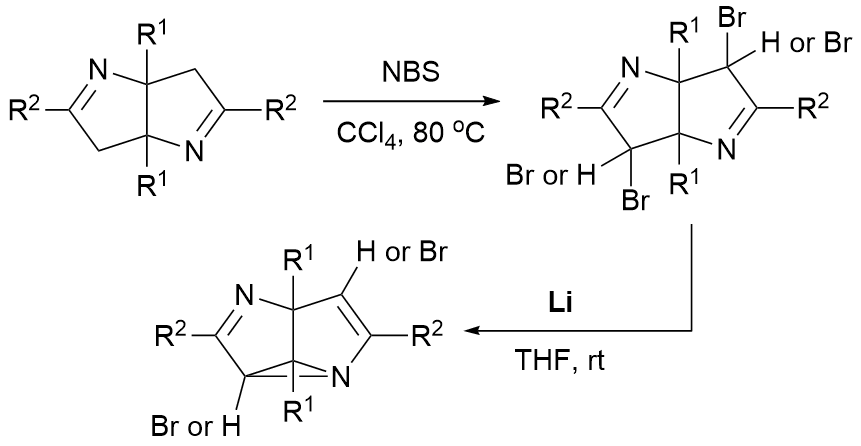
Treatment of Δ1-dipyrrolines with NBS afforded α,α’-dibromo-Δ1-bipyrrolines and α,α,α’,α’-tetrabromo-Δ1-bipyrrolines respectively with excellent selectivity depending on the amount of NBS. All these multibromo-substituted Δ1-bipyrrolines could be efficiently transformed into their corresponding 2,6-diazasemibullvalene derivatives via reduction with lithium. An unprecedented rearrangement of 4,8-dibromo-2,6-diazasemibullvalene afforded a new type of bipyrroline derivative.
2,6-Diazasemibullvalenes (NSBVs) have attracted fundamental interest both theoretically and experimentally for a long time because of their rapid aza-Cope rearrangement and the predicted existence of a homoaromatic delocalized structure. However, synthesis and structural study of NSBV derivatives have been a great challenge in organic chemistry. On the other hand, Δ1-bipyrroline derivatives are a class of important compounds of interesting structure. N-Containing fused-ring is a common moiety in synthetic intermediates and biologically active compounds. While synthetic methods for Δ1-bipyrrolines are rare, we have found that the reaction of dilithio reagents with nitriles is an efficient way (Chem.-Eur. J., 2008, 14, 5670). Herein, based on the synthetic method of Δ1-bipyrrolines developed by our lab, we could largely expand the scope of NSBV derivatives. A number of 3,7-dialkyl-substituted and 3,7-diaryl-substituted NSBVs could be obtained in good to excellent yields.
