Research news, Jan. 19th, 2018
Lewis Acid-Promoted Ring-Contraction of 2,4,6,8-Tetrasubstituted
1,5-Diazacyclooctatetraenes to 2,4,6-Trisubstituted Pyridines
Zhe Huang, Wen-Xiong Zhang, and Zhenfeng Xi*
Org. Lett. 2018, 20, 485-488.
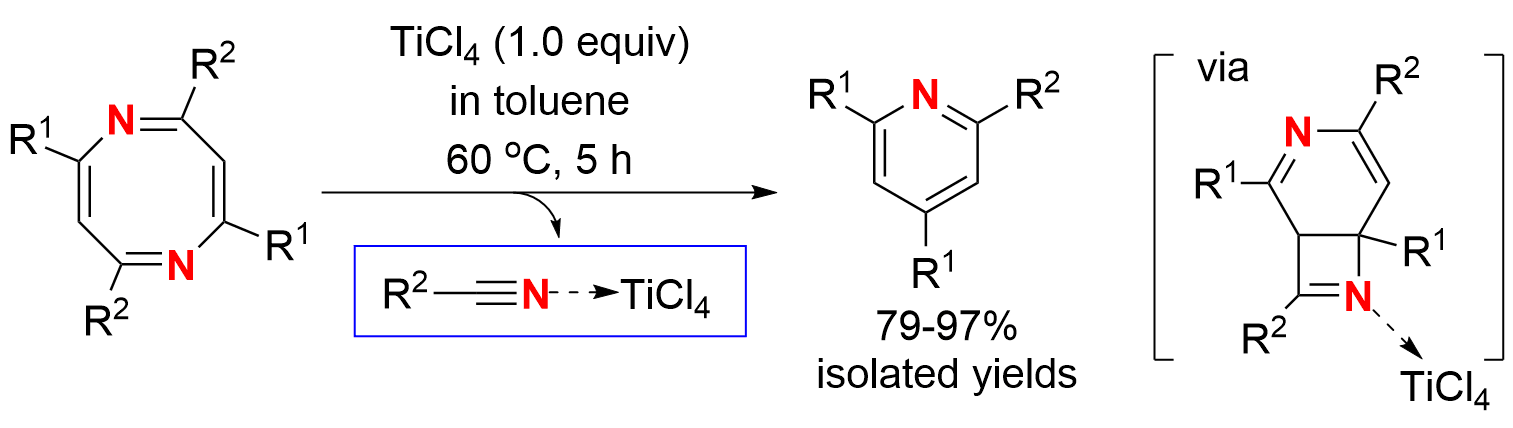
Ring-contraction reactions are synthetically useful methods in organic synthesis for making smaller rings from larger rings. While a number of anion, cation and carbenoid ring-contractions have been well documented and applied, ring-contractions via intramolecular pericyclic protocol are less explored and often not useful for synthesis. For example, cyclooctatetraene (COT) could undergo ring-contraction to benzene under photo-irradiation condition or thermal condition, but isomerization of COT to styrene took place at the same time and resulted in mixed products. The ring-contraction of 2-methoxy-azacyclooctatetraene in the presence of base generated benzonitrile in a moderate yield. Herein, we report a useful ring-contraction of 2,4,6,8-tetrasubstituted 1,5-diazacyclooctatetraenes (NCOTs for short) to 2,4,6-trisubstituted pyridines with excellent yields under mild condition by treatment with TiCl4. In this process, an intramolecular 6π electrocyclic ring-closing of NCOT was followed by Lewis acid mediated retro [2 + 2] cyclization.
NCOT (1,5-diazacyclooctatetraene) is interesting analogues of COT (cyclooctatetraene), but its reactivity remains almost entirely unknown except the reduction with alkali metals. We have developed a one-pot synthetic route to NCOTs from 1,4-dilithio-1,3-dienes and nitriles (Zhang, S.; Wei, J.; Zhan, M.; Luo, Q.; Wang, C.; Zhang, W.-X.; Xi, Z. J. Am. Chem. Soc. 2012, 134, 11964; Zhang, S.; Zhang, W.-X.; Xi, Z. Acc. Chem. Res. 2015, 48, 1823.). It is supposed that the introduction of N atoms into the conjugated system may bring about new reactivity as Schiff base, so we carried out reactions of NCOTs with Lewis acids.
