Research news, Aug. 15th, 2015
1,3-Butadienyl Dianions as Non-innocent Ligands: Synthesis and Characterization of Aromatic Dilithio Rhodacycles
Junnian Wei, Yongliang Zhang, Wen-Xiong Zhang, and Zhenfeng Xi*
Angew. Chem. Int. Ed. 2015, 54, 9986-9990.(Very Important Paper)
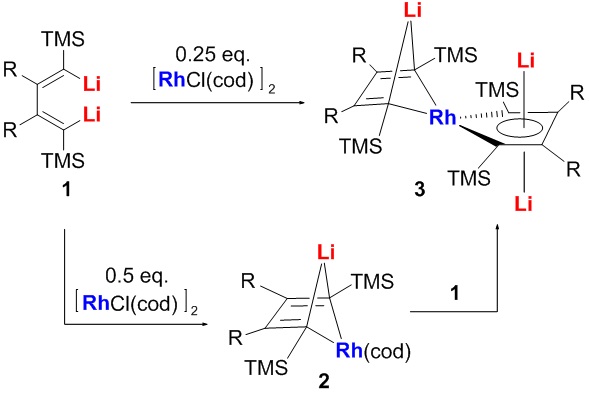
Recently we reported the first example that organolithium reagents might behaved as formal oxidants (Angew. Chem. Int. Ed. 2015, 54, 5999-6002). In that reaction, 1,4-dilithio-1,3-butadienes (dilithio reagents for short) reacted with Ni(cod)2, offering aromatic dilithionickeloles as final products. Ni(0) was assumably oxidized to Ni(II) by the dilithio reagents based on XPS data. Excited by this fascinating discovery and at the meantime as our conclusion seems to have conflicts with classic theory of formal oxidation state formalism, we tried to have more examples and evidences to discuss this topic more deeply and systematically.
Here we report that 1,4-dilithio-1,3-butadienes, a type of 1,3-butadienyl dianions, can act as non-innocent ligands, taking electrons from low-valent transition metals. Dilithio reagents reacted with [RhCl(cod)]2 to offer dilithio rhodacycle. Single-crystal X-ray structural analysis revealed the structure with averaged bond lengths. XPS data suggested that the oxidation state of Rh was more likely to be Rh3+. CDA/ECDA confirmed the electron transfer process. 7Li NMR spectra and theoretical calculations revealed a considerable aromatic character. In this process, the dilithio compounds behaved as non-innocent ligands and formal oxidants. These results further demonstrated that organolithium compounds with suitable π-conjugation could be used as electron acceptor.
