Research news, Sept. 24th, 2015
Combining Pd(p-allyl)Cp and PPh3 as a unique Catalyst for Efficient Synthesis of Alkyliodo Indoles via C(sp3)-I Reductive Elimination
Wei Hao, Han Wang, Patrick J. Walsh* and Zhenfeng Xi*
Org. Chem. Front. 2015, 2, 1080-1084.
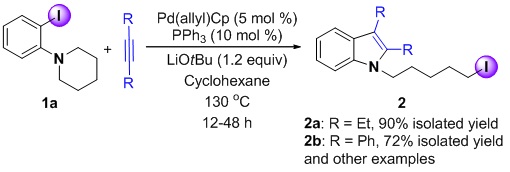
Alkyl iodides are important reagents in synthetic organic chemistry and their synthesis has attracted much attention. Traditionally, they are prepared by the reaction of alcohols with I2/PPh3 or by conversion of alcohols to sulfonates followed by treatment with NaI. Despite the widespread use of such reactions, new methods to prepare these classic electrophiles with increasing degrees of functionality remain in demand.
Recently it has been shown that alkyl iodides can be prepared using transition metal catalysts via an unusual reductive elimination pathway. The C–I bond-forming reductive elimination step takes place from an intermediate LnM(alkyl)(I) that can also undergo facial b-hydride elimination, limiting the synthetic utility of this approach. As a consequence, the alkyl groups of [LnM(alkyl)(I)] intermediates usually do not contain syn-b-hydrogen atoms to circumvent generation of alkene byproducts.
In this work, the combination of Pd(p-allyl)Cp and PPh3 was found to generate an efficient catalyst for the formation of indole-containing alkyl iodides from ortho-amino iodobenzenes and both aromatic and aliphatic alkynes. A unique feature of this reaction is a palladium-promoted C(sp3)-I bond formation via reductive elimination. This catalytic process was found to be very sensitive to the size and equivalents of phosphine ligands and the nature of the base.
