Research news, Jan. 21st, 2016
Aromatic Dicupra[10]annulenes
Junnian Wei, Yongliang Zhang, Yue Chi, Liang Liu, Wen-Xiong Zhang, and Zhenfeng Xi*
J. Am. Chem. Soc. 2016, 138, 60−63.
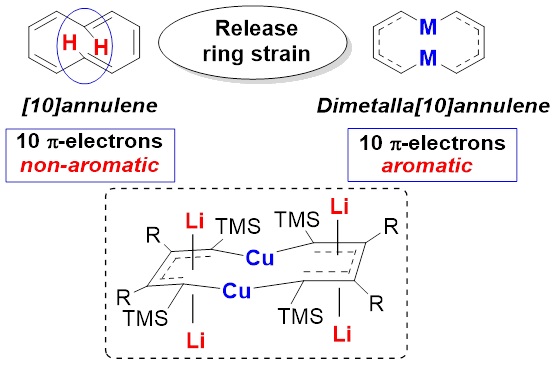
BACKGROUND: The chemistry of metal-containing aromatics (metalla-aromatics) has long been a fundamental and fascinating topic. However, the synthesis of macrocyclic metalla-aromatics with different metals still remains a great challenge. On the other hand, annulenes, which usually have a general formula C2nH2n, are classic cyclic conjugated systems. However, the [10]annulene having 10 π-electrons is non-aromatic, due to the steric hindrance of the two internal hydrogens. To release the steric hindrance of [10]annulene, we considered to replace the two internal C-H fragments with transition metals. Thus, if each of the two metals could offer one electron to form delocalized π-bonds, then the dimetalla[10]annulene could be aromatic. We have been working on the synthetic applications of multiply substituted 1,4-dilithio 1,3-butadienes (dilithio reagents for short). Recently, we found that appropriate dilithio reagents could react with low-valent transition metals, offering aromatic dilithio-metalloles (Wei, J.; Zhang, W.-X.; Xi, Z. Angew. Chem., Int. Ed. 2015, 54, 5999; Wei, J.; Zhang, Y.; Zhang, W.-X.; Xi, Z. Angew. Chem., Int. Ed. 2015, 54, 9986.), in which the dilithio reagents behaved as non-innocent ligands. Inspired by the novel behavior of dilithio reagents, we envisioned that macrocyclic metalla-aromatics could be obtained by annulating two or more dilithio reagents with suitable transition metals. This work reports the synthesis, characterization, and theoretic study of macrocyclic metalla-aromatics, dicupra[10]annulenes.
ABSTRACT: Metal-containing aromatic systems (metalla-aromatics) are unique and important both experimentally and theoretically. Among metalla-aromatics, 6-membered metallabenzenes and metallabenzynes have attracted much attention in recent years. However reports on their superior homologues are rare. In this work, the first series of aromatic dicupra[10]annulenes were isolated from the reaction of dilithio reagents and copper salts. Single-crystal X-ray structural analysis revealed dicupra[10]annulenes with averaged bond lengths. 7Li NMR spectra and theoretical calculations revealed a considerable aromatic character. XPS data suggested that the oxidation state of Cu atom in dicupra[10]annulenes was more likely to be Cu(I), indicating that the dilithio moieties in dicupra[10]annulenes were participated as non-innocent ligands. This work demonstrates a novel approach to construct macrocyclic metalla-aromatics.
