Research news, Dec. 4th, 2015
Half-sandwich rare-earth metal tris(alkyl) ate complexes catalyzed phosphaguanylation reaction of phosphines with carbodiimides: an efficient synthesis of phosphaguanidines
Wangyang Ma, Ling Xu, Wen-Xiong Zhang,* and Zhenfeng Xi
New J. Chem. 2015, 39, 7649-7655.
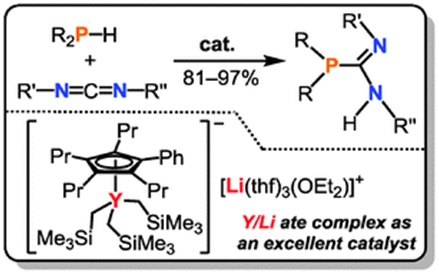
The catalytic guanylation reaction of amines with carbodiimides (CGAC reaction) has become a hot area of research, because it provides a straightforward and atom-economical route to prepare guanidines. Phosphaguanidines, as analogues of guanidines, are widely used as building blocks of ancillary ligands for many transition metal species.
In this work, we have reported a half-sandwich yttrium tris(trimethylsilylmethyl) ate complex catalyzed phosphaguanylation reaction of phosphines with carbodiimides to efficiently prepare phosphaguanidines. The present yttrium catalyst system is easier to prepare and cheaper than those reported using neutral lanthanum complexes. It displays the best catalytic activity among the known yttrium complexes probably owing to the cooperative effect between the anionic yttrium core and cationic lithium moiety.
