Research news, Jun. 15th, 2015
Semibullvalene and Diazasemibullvalene: Recent Advances in the Synthesis, Reaction Chemistry, and Synthetic Applications
Shaoguang Zhang, Wen-Xiong Zhang, and Zhenfeng Xi*
Acc. Chem. Res. 2015, DOI:10.1021/acs.accounts.5b00190
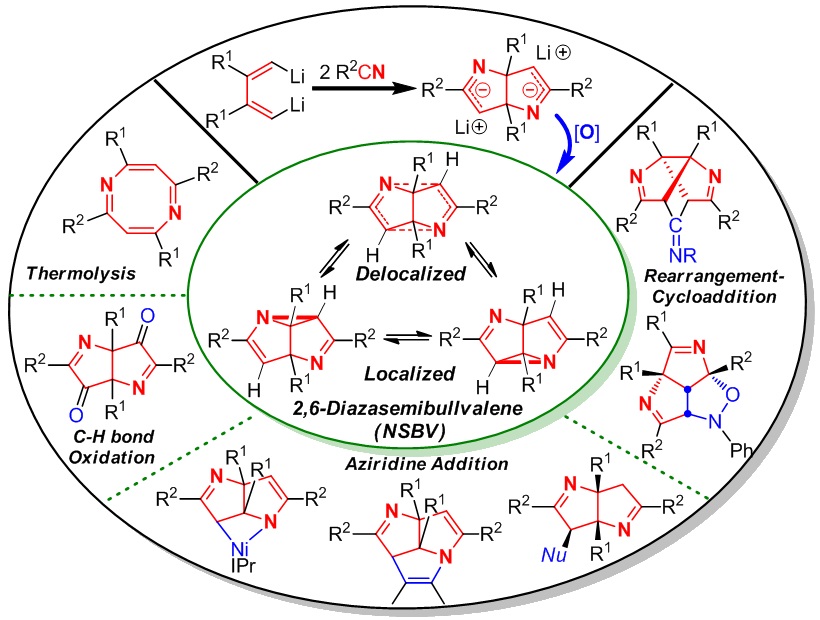
Semibullvalene (SBV) and its aza-analogue 2,6-diazasemibullvalene (NSBV) are both theoretically interesting and experimentally challenging organic molecules because of four unique features: highly strained ring systems, intramolecular skeletal rearrangement, extremely rapid degenerate (aza)Cope rearrangement, and predicted existence of neutral homoaromatic delocalized structures. SBV has received much attention in the past 50 years. In contrast, since NSBV was predicated in 1971 and the first in situ synthesis was realized in 1982, no progress on NSBV chemistry was made until our results in 2012. We have been interested in reaction chemistry of 1,4-dilithio-1,3-butadienes (dilithio reagents for short), especially for its application in synthesis of SBV and NSBV. This is because: i) the cyclodimerization of dilithio reagents could provide the potential 8-carbon skeleton of SBV from butadiene 4-carbon unit, and ii) the insertion reaction of dilithio reagents with C≡N bonds of two nitriles could provide the (6-C+2-N) skeleton which might be a good precursor for the synthesis of NSBV. Therefore, we initiated a journey to the synthesis and reaction chemistry of SBV and NSBV starting from dilithio reagents since 2006. In this Account, we outline mainly our recent achievement on the synthesis, structural characterization, reaction chemistry, synthetic application, and theoretical/computational analysis of NSBV.
Two efficient synthetic strategies for NSBV from dilithio reagents and nitriles via oxidant-induced C-N bond formation will be introduced. The structural investigation of NSBV, including X-ray crystal structure, determination of the activation barrier of aza-Cope rearrangement, and theoretical analysis, shows that the localized structure of NSBV is the predominant form, and the homoaromatic, delocalized structure exists as a minor component in the equilibrium. Then we will introduce the reaction chemistry and synthetic applications of NSBV. Several novel reaction patterns were explored including thermolysis, C-N bond insertion, rearrangement-cycloaddition, oxidation and nucleophilic ring-opening reaction. Diverse and interesting N-containing polycyclic skeletons could be constructed, such as nickelaazetidine, 1,5-diazatriquinacenes and triazabrexadienes, which are not available by other means.
Our results show that NSBV not only features rapid (aza)Cope rearrangement with low activation barrier, but also acts as unique synthetic reagent, which is significantly different from aziridine. The strained rigid ring systems as a whole could be involved in the reactions. Our achievement highlights two significant progresses: i) The well-established efficient synthesis and isolation of NSBV greatly accelerate the development of NSBV chemistry, and ii) The previously unattainable molecules have become “normal” and routine starting materials for the synthesis of otherwise unavailable but interesting structures. We expect our pursuits will inspire and help direct future efforts on chemical and physical research of NSBV.
