Research news, Jun. 3rd, 2015
Mechanistic Considerations of the Catalytic Guanylation Reaction of Amines with Carbodiimides for Guanidine Synthesis
Ling Xu, Wen-Xiong Zhang,* and Zhenfeng Xi
Organometallics 2015, 34, 1787−1801 (Review).
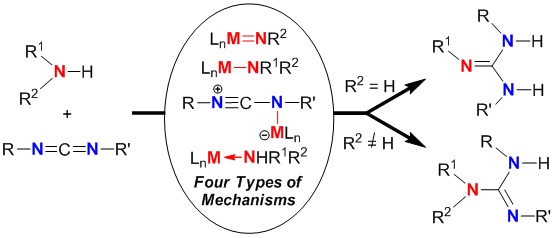
Catalytic guanylation reaction of amines with carbodiimides has received the increasing attention because of the atom-economical preparation of guanidines since 2003. To date, more than forty catalysts including transition metals, main group and rare-earth metals have been designed and tested for the guanylation reaction to construct acyclic and cyclic guanidines. In this review, we present a mechanistic consideration on catalytic guanylation reaction of amines with carbodiimides for guanidine synthesis to elucidate its development and importance. Four different types of reaction mechanisms are well categorized: [2+2]-cycloaddition/protonation mechanism, insertion/protonation mechanism, activation of carbodiimide/nucleophilic addition/intramolecular protonation mechanism, and protonation/nucleophilic addition/disassociation mechanism. It is useful to understand this reaction processes and accelerate the rapid development in the related area to meet the needs of the growing guanidines.