Research news, Mar. 19th, 2015
Dianions as Formal Oxidants: Synthesis and Characterization of Aromatic Dilithionickeloles from 1,4-Dilithio-1,3-butadienes and Ni(COD)2
Junnian Wei, Wen-Xiong Zhang, and Zhenfeng Xi*
Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201411009
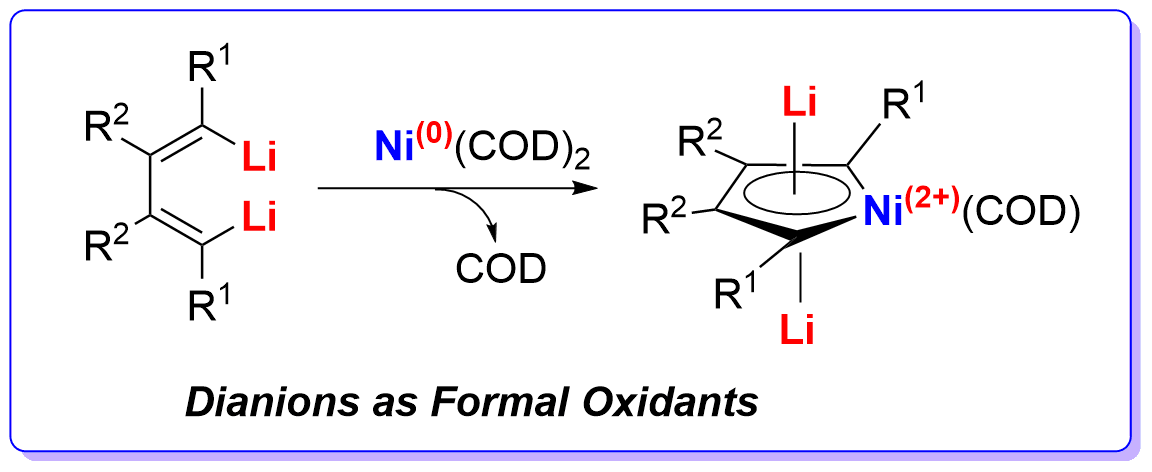
Organolithium compounds can behave as reductants but never as oxidants in redox reactions. Here we report that 1,4-dilithio-1,3-butadienes reacted with Ni(COD)2 to offer dilithionickeloles. Single-crystal X-ray structural analysis revealed a coplanar structure of dilithionickeloles with averaging of bond lengths. XPS data confirmed the oxidation state of Ni in dilithionickeloles was Ni2+. 7Li NMR spectra of dilithionickeloles and theoretical calculations revealed a considerable aromatic character. In this redox reaction, the dilithio dianionic compounds behaved as formal oxidants, oxidizing the Ni0 atom to Ni2+. These results demonstrated that organolithium compounds with π-conjugation could be used as oxidants and could continue to gain extra electrons.
