Research news, Aug. 27th, 2014
Synthesis and Applications of 1-Iodo-4-MgCl-1,3-dienes and 1-Iodovinyl Phenylmagnesium Chlorides
Junnian Wei, Yongliang Zhang, Wen-Xiong Zhang and Zhenfeng Xi*
Org. Chem. Front. 2014, DOI: 10.1039/c4qo00191e
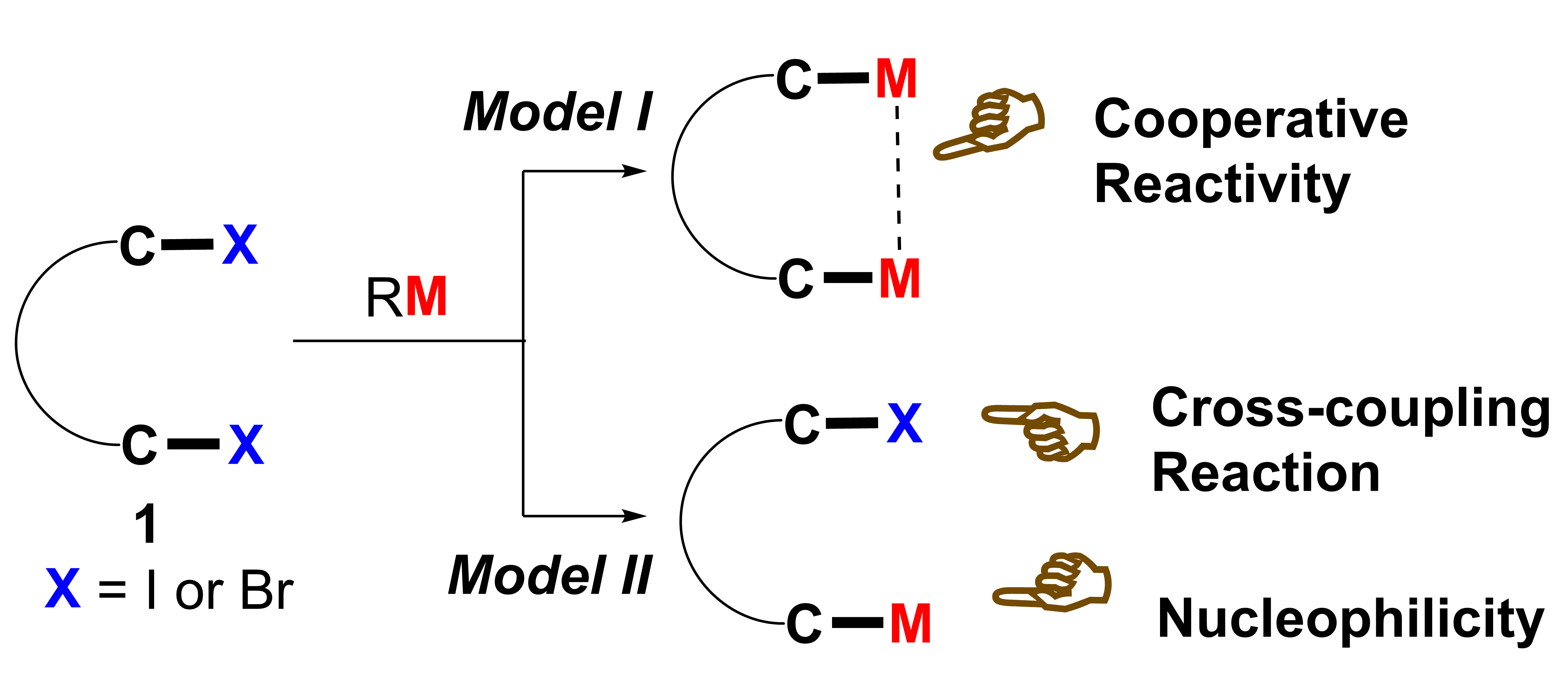
Dihalo compounds have become readily available. From such dibromides or diiodides, their corresponding organo-di-metallic compounds (reagents), including 1,4-dilithio-1,3-dienes, 1,4-dicopper-1,3-dienes and 1,4-dimagsia-1,3-dienes, can be easily generated (Model I). Reaction chemistry study of these organo-di-metallic reagents has demonstrated unique and useful synthetic applications because of the cooperative effect of the two C-M bonds in the same molecule. Conceptually, if one of the two C-X bonds could be selectively metalated, compounds containing one C-X bond and one C-M bond in the same molecule would be generated (Model II). Useful synthetic applications of such compounds can be thus expected because the C-X bond and the C-M bond will react with totally different reagents. For example, the C-M bond will react with various electrophiles while the C-X bond can be applied to numerous cross-coupling reactions.
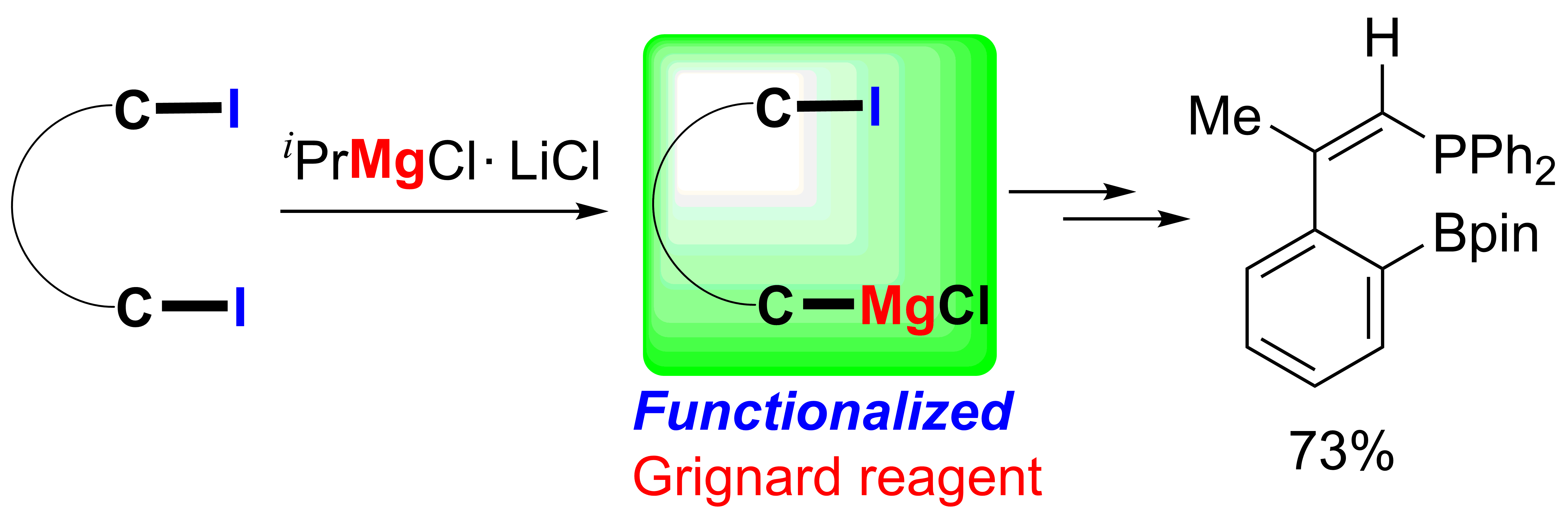
Selective I/Mg exchange reactions of 1,4-diiodo-1,3-dienes and o-iodo-2-(2-iodovinyl)benzenes were achieved via iPrMgCl•LiCl. Various 1-iodo-4-MgCl-1,3-dienes and 1-iodovinyl phenylmagnesium chlorides were thus efficiently generated and synthetically applied to afford new C-C bonds via reactions of the alkenyl or aryl C-MgCl bonds with different electrophiles. Useful conjugated compounds including polysubstituted benzenes, naphthalenes and phosphine compounds could be synthesized readily via further applications of the remaining alkenyl C-I bonds and subsequent cyclization reactions.
