Dual-Function Tetrabenzylphosphonium Groups as Mitochondria Targeting Artificial Anion Channels
Fei Gou#, Xinlei Huangfu#, Qiuting Wang, Zihong Yang, Xiyu Yuan, Wenju Chang, Jie Shen*, Wen-Xiong Zhang*, and Huaqiang Zeng*
Angew. Chem. Int. Ed. 2025, 64, e202511936. ( #Authors contributed equally)
https://doi.org/10.1002/anie.202511936
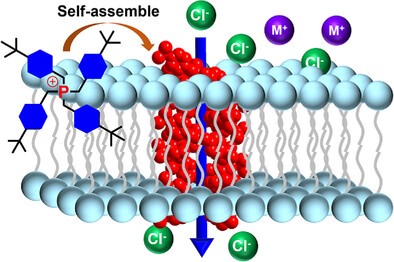
Artificial ion channels with specific organelletargeting capabilities have been scarcely investigated. Here, we report the first-in-class mitochondria-targeting anion channels derived from a structurally simple tetrabenzylphosphonium framework, in stark contrast to its phenyl-based counterpart, which lacks anion transport activity. Structural and computational analyses underscore the critical role of the methylene (CH2) linkers in the benzyl groups. These CH2 units reduce positive charge delocalization to enhance σ-hole–anion interactions, while also enabling H-atoms from both the CH2 linkers and aromatic rings to cooperatively form multiple C─H···anion H─bonds. In further conjunction with the rigid benzene rings, they help create sufficient spatial voids to accommodate anion translocation, collectively facilitating and energizing the anion transport process. Among the series studied, those bearing methyl and tertbutyl substituents exhibit the highest transport activity via a channel mechanism, with a conductance value as high as 26.5 ± 0.8 pS. Furthermore, leveraging the cationic nature of the quaternary phosphonium center, this family of anion channels readily achieves targeted mitochondrial localization, demonstrating potent anticancer activity, with IC50 values ranging from 1.42 to 3.04 µM across three cancer cell lines.




