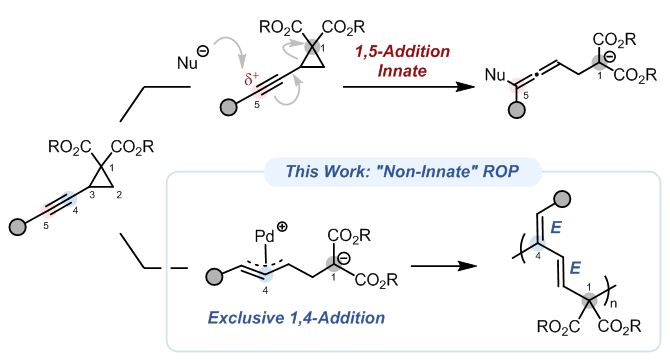
In this work, we report catalyst-controlled, non-innate connectivity in the ROP of alkynylcyclopropane dicarboxylates (ACPs). Leveraging π-propargyl palladium intermediates, we redirect the reactivity from the electronically favored 1,5-addition to a non-innate 1,4-addition pathway, furnishing a conjugated diene-based backbone with high regio- and stereoselectivity. The polymerization can be controlled at low monomer-to-initiator ratios, enabling block copolymer synthesis with well-defined chain ends.