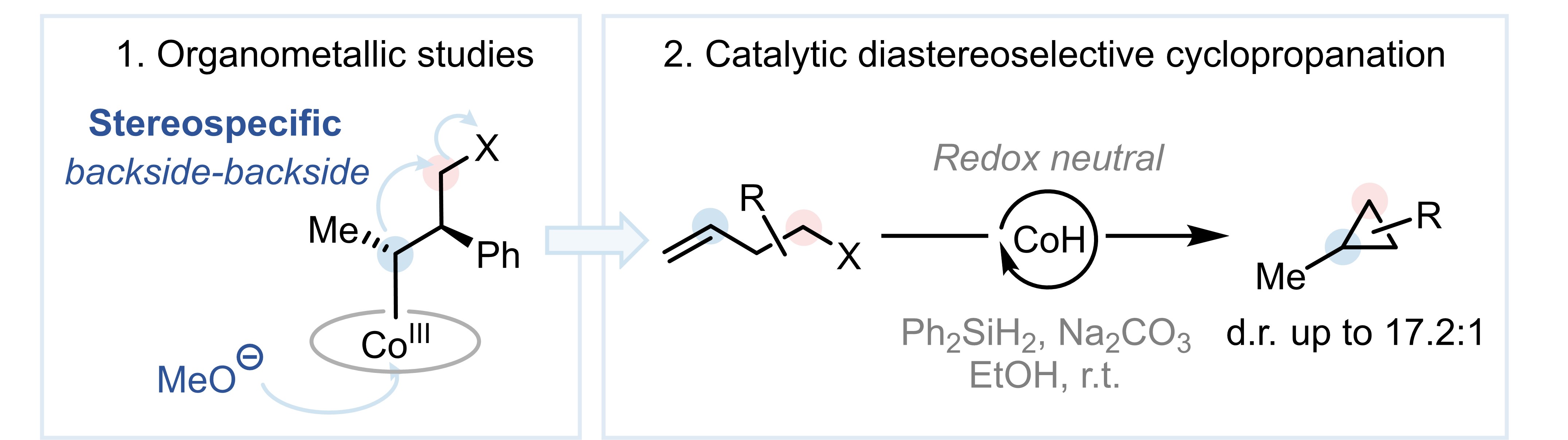
In this work, we report an axial-coordination activation strategy to unlock nucleophilic reactivity space in Co-MHAT catalysis. Through stoichiometric organometallic studies using well-defined alkyl–CoIII complexes, we demonstrate that an alkoxide ligand can render alkyl−CoIII sufficiently nucleophilic to displace a suitable leaving group, via a stereospecific backside-backside approach. This pathway is further applied in catalysis, enabling a diastereoselective redox neutral Co-MHAT cyclopropanation reaction.