Allostery of Multidomain Proteins
时间:2020-12-31
Qiaojing Huang, Maodong Li, Luhua Lai*, and Zhirong Liu*.
Allostery of multidomain proteins with disordered linkers.
Curr. Opin. Struct. Biol. 62, 175-182 (2020).
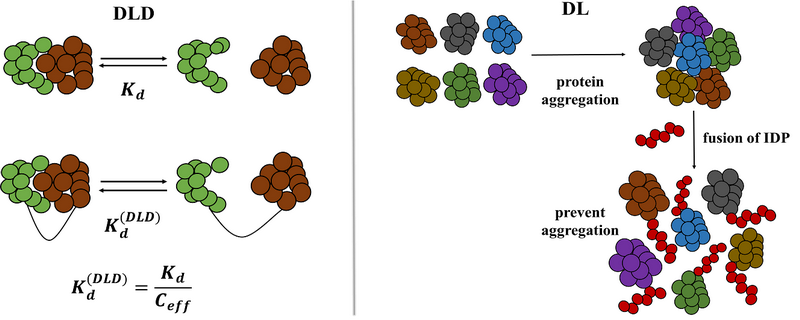
Abstract: Intrinsically disordered regions are often involved in allosteric regulation of multidomain proteins. They can act as disordered linkers to connect and interact with domains, resulting in rather complex allosteric mechanism and novel protein behavior. Therefore, it is necessary to analyze the diverse functions of disordered linkers in order to better understand allostery and relevant regulation process. Here we summarize recent advances in understanding the function of linkers and the advantages of adopting mutlidomain architecture with disorder linkers. It was shown that linkers between domains enhance the local domain concentration and make the allosteric regulation of weakly interacting partners possible, while linkers with only one tethered end cause an entropy effect to reduce binding affinity and prevent aggregation.
Link: https://www.sciencedirect.com/science/article/pii/S0959440X20300178




