Advantages of IDPs
时间:2020-12-30
A widely discussed property of intrinsically disordered proteins (IDPs) is that they may have a higher binding rate in molecular binding (which will facilitate signal transduction). A well-known model in this topic is the fly-casting mechanism [Proc. Natl. Acad. SCI. U.S.A. 97, 8868 (2000)] proposed by Peter G. Wolynes et al. It was believed that the disordered protein can bind to the target faster because of its larger capture radius. However, the applicability of this mechanism is controversial. We developed a coarse-grained model to study this problem. The results showed that although disordered proteins have larger capture radius, they have smaller diffusion coefficient, so the capture speed of disordered proteins is not higher than that of ordered proteins; the real reason why disordered proteins have kinetic advantages is that they are easier to evolve into the final binding structure after being captured; in addition, it is found that structural flexibility helps proteins enhance their ability to resist environmental changes. The mechanism of "dynamic buffer effect" is proposed accordingly.
This work was published in the Journal of Molecular Biology in 2009:
Yongqi Huang and Zhirong Liu*. Kinetic advantage of intrinsically disordered proteins in coupled folding-binding process: a critical assessment of the "fly-casting" mechanism. J. Mol. Biol. 393 (5), 1143-1159 (2009).
After the publication of the work, it was recommended by V. N. Uversky, an expert in the field, in the Faculty of 1000 biology, and was evaluated as "This is a very interesting, important, and provocative study which will be of interest to everyone studying protein interactions", link: http://f1000biology.com/article/id/1226965/evaluation. The November 2009 issue of the Biophysical Society newsletters also selected this work into the Papers of Interest.
In 2014, we were invited to writea a review article entitled "Advantages of proteins being disordered" (Zhirong Liu* and Yongqi Huang, Protein. Sci. 23 (5), 539-550 (2014))。
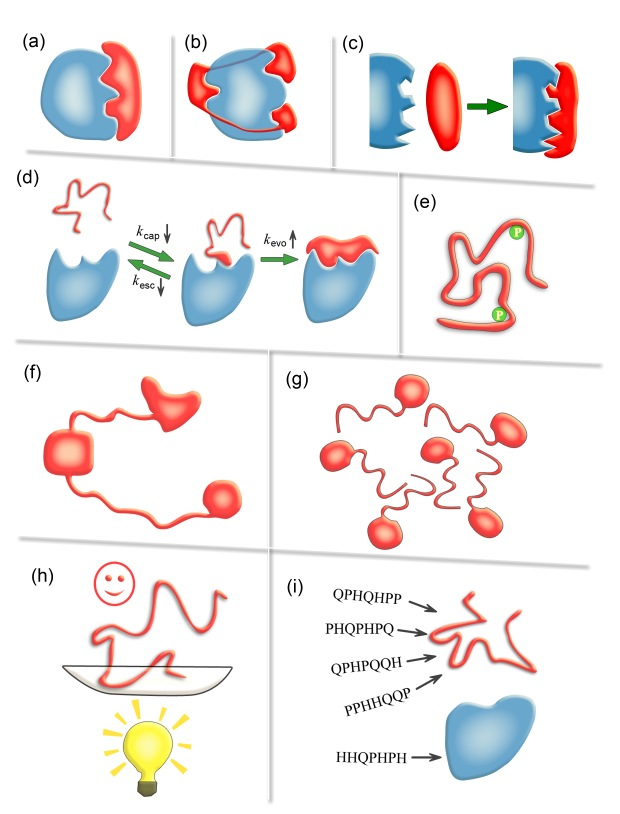
Schematic diagrams illustrating nine possible advantages of IDPs/IDRs. (a) Economizing genome and protein resources: IDPs/IDRs use smaller protein size to afford the same interface area as ordered proteins. (b) Overcoming steric restrictions in binding: IDPs/IDRs can overcome steric restrictions in binding complexes by protruding into partners or wrapping around them in ways difficult for ordered proteins. (c) Achieving high specificity with low affinity: the highly complementary binding interfaces and the unfavorable conformational-entropy changes result in high specificity and low affinity. (d) Increasing binding rate: the kinetic advantage of IDPs in a binding process stems from a faster evolution step from the encounter complex and a slower escaping rate. (e) Facilitating posttranslational modifications: the conformational flexibility of IDPs/IDRs greatly facilitatesthe exposure of modification sites and their binding to modifying enzymes. (f) Enabling flexible linkers: the lack of ordered structures makes IDPs/IDRs dominant in flexible linkers of proteins which are obviously out of reach of ordered proteins. (g) Preventing aggregation: IDPs/IDRs possess favorable interactions with water and are inherently advantageous in preventing aggregation. (h) Providing resistance to non-native conditions: you cannot unfold what is already unfolded. (i) Allowing compatibility with more available sequences: the sequence space of IDPs/IDRs is expected to be larger than that of ordered proteins.
Links:
[1] Yongqi Huang and Zhirong Liu*. Kinetic advantage of intrinsically disordered proteins in coupled folding-binding process: a critical assessment of the "fly-casting" mechanism. J. Mol. Biol. 393 (5), 1143-1159 (2009)
[2] Zhirong Liu* and Yongqi Huang. Advantages of proteins being disordered. Protein. Sci. 23, 539 (2014).




