研究室工作进展 Mar. 3rd, 2018
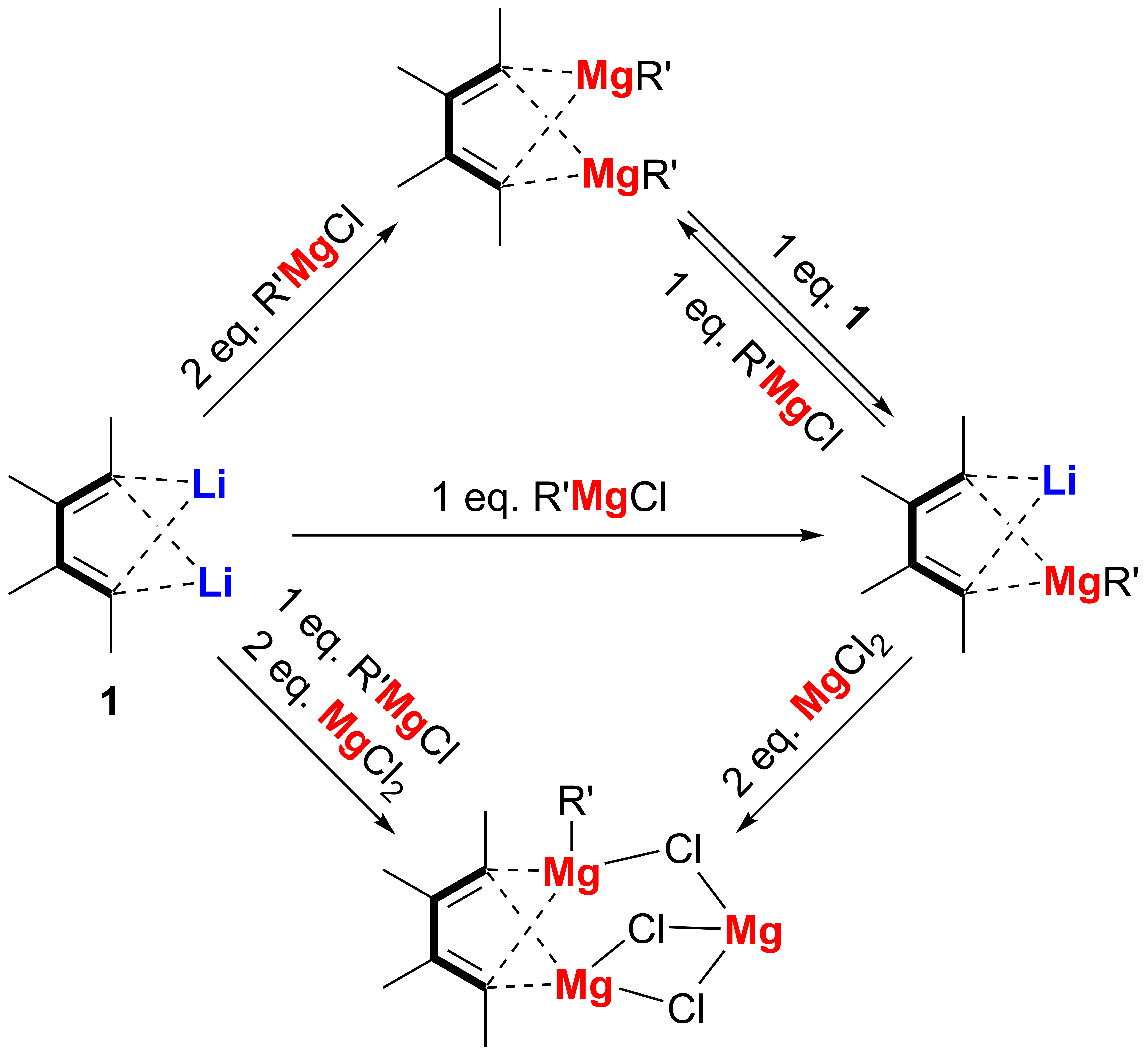

Transmetalation is a powerful approach to prepare organometallic compounds. Based on 1,4-dilithio 1,3-butadienes (dilithio reagent for short) and their transmetalation process, we have successfully obtained a variety of butadiene-based organometallic compounds containing one or more alkenyl-metal bonds, which are usually not readily available. In 2014, we reported the synthesis and structural characterization of the first series of magnesiacyclopentadienes, spiro dilithio magnesiacyclopentadienes and dimagnesiabutadienes from dilithio reagents. Although organomagnesium reagents including lithium organomagnesiates are widely applied in synthetic chemistry, however, the study and characterization of structurally well-defined organomagnesium compounds including lithium organomagnesiates, especially in cases of those compounds with alkenyl magnesium bonds, remain a great challenge, mainly due to the lack of synthetic methods and frequently occurred Schlenk equilibrium.
Thus, as our continued interest in the synthesis and structural elucidation of organomagnesium compounds, in this work, a series of butadienyl organomagnesium complexes, including 1-Li-4-(MgR)-butadienes, 1,4-bis(MgR)-butadienes, and 1-Mg-4-(MgR)-butadiene were synthesized starting with 1,4-dilithio butadienes via the transmetalation reaction between alkenyllithium bond and alkenylmagnesium bond. Single-crystal X-ray structural analysis of these butadienyl organomagnesiates revealed unique bonding modes. Study on their structural transformation demonstrated that 1-Li-4-(MgR)-butadiene is the key intermediate in the reaction process leading to the formation of 1,4-bis(MgR)-butadiene and 1-Mg-4-(MgR)-butadiene. All these results demonstrated that transmetalation process from C(sp2)-Li bonds to C(sp2)-Mg bonds can be selectively tuned, leading to structural diversity of the main group organometallic reagents.
