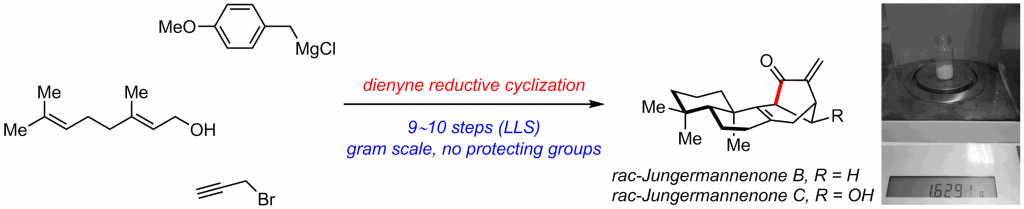
We report the first scalable total synthesis of tetracyclic diterpenoid jungermannenones B and C
Reported is the first scalable synthesis of?rac-jungermannenones?B and C starting from the commercially available and inexpensive geraniol in 10 and 9 steps, respectively. The unique jungermannenone framework is rapidly assembled by an unprecedented regioselective 1,6-dienyne reductive cyclization reaction which proceeds through a vinyl radical cyclization/allylic radical isomerization mechanism. DFT calculations explain the high regioselectivity observed […]
Reported is the first scalable synthesis of?rac-jungermannenones?B and C starting from the commercially available and inexpensive geraniol in 10 and 9 steps, respectively. The unique jungermannenone framework is rapidly assembled by an unprecedented regioselective 1,6-dienyne reductive cyclization reaction which proceeds through a vinyl radical cyclization/allylic radical isomerization mechanism. DFT calculations explain the high regioselectivity observed in the 1,6-dienyne reductive radical cyclization.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201511659/full